
Carbamic acid, [imino(methylthio)methyl]-, 1,1-dimethylethyl ester (9CI) synthesis
- Product Name:Carbamic acid, [imino(methylthio)methyl]-, 1,1-dimethylethyl ester (9CI)
- CAS Number:173998-77-1
- Molecular formula:C7H14N2O2S
- Molecular Weight:190.26

24424-99-5
821 suppliers
$13.50/25G

2986-19-8
67 suppliers
inquiry
![Carbamic acid, [imino(methylthio)methyl]-, 1,1-dimethylethyl ester (9CI)](/CAS/GIF/173998-77-1.gif)
173998-77-1
41 suppliers
$40.00/100mg
![tert-butyl (NZ)-N-[[(2-methylpropan-2-yl)oxycarbonylamino]-methylsulfanylmethylidene]carbamate](/CAS/20180808/GIF/322474-21-5.gif)
322474-21-5
10 suppliers
inquiry
Yield: 55% , 15%
Reaction Conditions:
with triethylamine;sodium hydroxide in dichloromethane at 0; for 4 h;
Steps:
2.1. N-tert-butoxycarbonyl-S-methylisothiourea (1a)
CH2Cl2 (5 ml) and triethylamine (TEA) (250 l, 1.79mmol) were added to a rapidly stirred solution of Smethylisothioureahemisulfate (500 mg, 3.59 mmol) inNaOH 1N (2.5 ml). The mixture was cooled to T=0°C on an ice bath, and a solution of di-tert-butyl dicarbonate (783.5mg, 3.59 mmol) in CH2Cl2 (2 ml) was added dropwise over 3h. Upon completion of the addition, the mixture was stirredan additional hour, diluted with 1 mL of CH2Cl2, and HCl 1Nwas added until pH 4. The organic layer was extracted withacidic water, and the aqueous layer was separated from theorganic phase and neutralized with NaOH 1N; CH2Cl2 (1 ml)was added and the aqueous phase was extracted twice. Theorganic layers were separated, dried over sodium sulfate,filtered and concentrated under reduced pressure. Diethylether was added and the solvent was evaporated under vacuumtwice. The compound purity was verified by HPLCanalysis as described above. The qualitative analysis of theproduct was finally confirmed by ESI-MS. The product wasthen used directly in the next step. (377 mg, 55 %)1H NMR (200 MHz, DMSO-d6): 1.39 (s, 9H), 2.45 (s,3H).13C NMR (50 MHz, DMSO-d6): 13.8, 27.8, 79.2, 157.3,161.4.ESI-MS m/z =191 [M+H] +
References:
Arkel, Maria;Garbati, Patrizia;Salis, Annalisa;Damonte, Gianluca;Liessi, Nara;Adriano, Enrico;Benatti, Umberto;Balestrino, Maurizio;Millo, Enrico [Medicinal Chemistry,2018,vol. 14,# 4,p. 1 - 7]

24424-99-5
821 suppliers
$13.50/25G
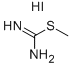
4338-95-8
50 suppliers
$22.00/1g
![Carbamic acid, [imino(methylthio)methyl]-, 1,1-dimethylethyl ester (9CI)](/CAS/GIF/173998-77-1.gif)
173998-77-1
41 suppliers
$40.00/100mg

24424-99-5
821 suppliers
$13.50/25G
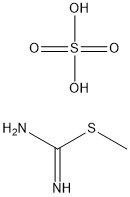
867-44-7
366 suppliers
$5.00/10g
![Carbamic acid, [imino(methylthio)methyl]-, 1,1-dimethylethyl ester (9CI)](/CAS/GIF/173998-77-1.gif)
173998-77-1
41 suppliers
$40.00/100mg

24424-99-5
821 suppliers
$13.50/25G

2986-19-8
67 suppliers
inquiry
![Carbamic acid, [imino(methylthio)methyl]-, 1,1-dimethylethyl ester (9CI)](/CAS/GIF/173998-77-1.gif)
173998-77-1
41 suppliers
$40.00/100mg