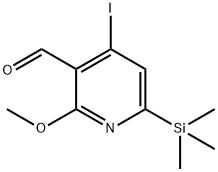
4-Iodo-2-Methoxy-6-triMethylsilanyl-pyridine-3-carbaldehyde synthesis
- Product Name:4-Iodo-2-Methoxy-6-triMethylsilanyl-pyridine-3-carbaldehyde
- CAS Number:174092-75-2
- Molecular formula:C10H14INO2Si
- Molecular Weight:335.21
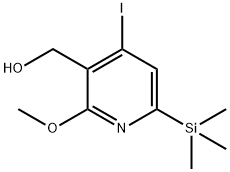
375346-05-7
1 suppliers
inquiry

174092-75-2
0 suppliers
inquiry
Yield:174092-75-2 87%
Reaction Conditions:
with sodium hypochlorite;2,2,6,6-Tetramethyl-1-piperidinyloxy free radical;sodium hydrogencarbonate in water;toluene at 0 - 5; for 0.5 h;
Steps:
18 Example 18; Synthesis of Compound (1) (2)
To a mixture of Compound (v) (1.00 g, content; 98.43%, 2.9 mmol), TEMPO (2.3 mg, 0.015 mmol, 0.005 eq.) and 7% (w/v) sodium hydrogen carbonate (7.0 mL) in toluene (8.7 mL), an aqueous solution of sodium hypochlorite (available chlorine; minimal 5%, 4.5 g, 3.0 mmol, 1.05 eq.) was added at 0 to 5 °C and then the mixture was sitirred at 0 to 5 °C for 2 hrs. 10% sodium sulf ite (3.7 mL, 2 . 9 mmol) was added to the mixture and the resulting mixture was stirred at 0 to 5 °C for 30 min. The insoluble material in the mixture was removed by filtration and the material on the filter paper was washed with toluene (1 mL × 3). The organic layer of the filtrate was separated and washed with water (10 mL), dried over sodium sulfate ( 2 g), filtered and the desiccant was washed with toluene. The filtrate and the washing were combined and then evaporated under reduced pressure to dryness. Compound (1) : yellowoil, 0.93g (87%yield), content: 90.60% by HPLC (see Example 17).
References:
EP1378505,2004,A1 Location in patent:Page 31
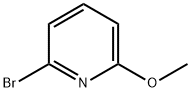
40473-07-2
275 suppliers
$7.00/1g

174092-75-2
0 suppliers
inquiry
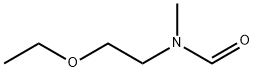
916851-24-6
0 suppliers
inquiry

174092-75-2
0 suppliers
inquiry
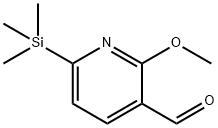
453518-21-3
0 suppliers
inquiry

174092-75-2
0 suppliers
inquiry

