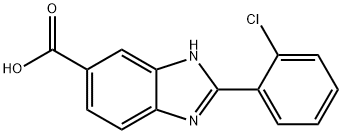
2-(2-Chloro-phenyl)-1H-benzimidazole-5-carboxylic acid synthesis
- Product Name:2-(2-Chloro-phenyl)-1H-benzimidazole-5-carboxylic acid
- CAS Number:174422-13-0
- Molecular formula:C14H9ClN2O2
- Molecular Weight:272.69
Yield:-
Reaction Conditions:
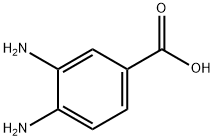
Stage #1: 2-chloro-benzaldehydewith sodium metabisulfite in ethanol;water at 20;
Stage #2: 3,4-diaminobenzoic acid in N,N-dimethyl-formamide at 130;
Steps:
6.1. General procedures for the preparation of 2-alkylbenzimidazole-5-carbohydrazides (4a-f) and 2-arylbenzimidazole-5-carboxylic acids (6a-l)
General procedure: To a solution of substituted benzaldehyde (1.50 mmol) in absolute EtOH (50 mL) was added dropwise sodium pyrosulfite (1.60 g, 8.00 mmol) in water (3 mL). The resulting solution was allowed to stir for 1-3 h at the room temperature and then for 3-4 h at 0 °C. The precipitate was collected and washed with EtOH to provide the addition products (5) as solids in yields ranging from 72% to 83%.A solution of 3,4-diaminobenzoic acid (0.62 g, 4.00 mmol) and the addition products 5 (2.00 mmol) in DMF (5 mL) was stirred at 130 °C for 6-12 h. After the completion of the reaction, the reaction solution was cooled to room temperature and added water (15 mL). The precipitate was collected and washed to give 2-arylbenzimidazole-5-carboxylic acids (6a-l). The yields were within the range of 68-75%. Some of the 2-substituted benzimidazole-5-carboxylic acid derivatives are as followed.
References:
Cong, Chao;Wang, Haiyang;Hu, Yue;Liu, Chen;Ma, Siti;Li, Xin;Cao, Jichao;Ma, Shutao [European Journal of Medicinal Chemistry,2011,vol. 46,# 7,p. 3105 - 3111] Location in patent:supporting information; experimental part