
ETHYL 2-CHLORO-3-CYANO-6-(TRIFLUOROMETHYL)-PYRIDINE-5-CARBOXYLATE synthesis
- Product Name:ETHYL 2-CHLORO-3-CYANO-6-(TRIFLUOROMETHYL)-PYRIDINE-5-CARBOXYLATE
- CAS Number:175277-73-3
- Molecular formula:C10H6ClF3N2O2
- Molecular Weight:278.61
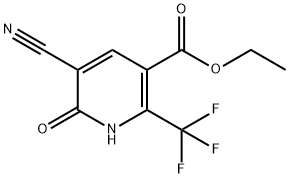
144456-86-0
13 suppliers
inquiry

175277-73-3
42 suppliers
$20.00/250mg
Yield:175277-73-3 95%
Reaction Conditions:
with oxalyl dichloride;N,N-dimethyl-formamide in dichloromethane at 50;Product distribution / selectivity;Heating / reflux;
Steps:
3.a; 5.a
Example 3Ethyl 6-(4-{[(benzylsulfonyl)amino]carbonyl}piperidin-l-yl)-5-cyano-2- 25 (trifluoromethyl)nicotinate(a) Ethyl 6-chloro-5-cyano-2-(trifluoromethyl)nicotinateOxalylchloride (12.20 g, 96.1 mmol) and DMF (0.744 mL) were added to a solution of 30 ethyl 5-cyano-6-oxo-2-(trifluoromethyl)-l,6-dihydropyridine-3-carboxylate (5 g, 19.22 mmol) (prepared essentially according to the method described in Mosti, L et al, Farmaco, VoI 47, No 4, 1992, pp. 427-437) and the reaction was heated to 5O0C over night. The
References:
WO2008/4946,2008,A1 Location in patent:Page/Page column 89-90; 93
571-55-1
156 suppliers
$10.00/1g

175277-73-3
42 suppliers
$20.00/250mg

372-31-6
468 suppliers
$5.00/10g

175277-73-3
42 suppliers
$20.00/250mg