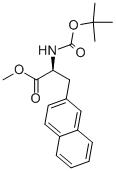
(S)-2-TERT-BUTOXYCARBONYLAMINO-3-NAPHTHALEN-2-YL-PROPIONIC ACID METHYL ESTER synthesis
- Product Name:(S)-2-TERT-BUTOXYCARBONYLAMINO-3-NAPHTHALEN-2-YL-PROPIONIC ACID METHYL ESTER
- CAS Number:176896-73-4
- Molecular formula:C19H23NO4
- Molecular Weight:329.39
Yield:176896-73-4 28%
Reaction Conditions:
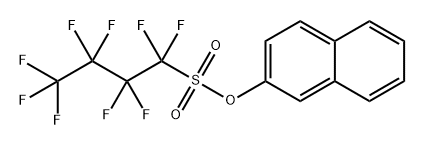
with nickel(II) bromide dimethoxyethane;manganese;bathophenanthroline;lithium bromide in N,N-dimethyl-formamide at 20 - 80;Inert atmosphere;
Steps:
2-Butyl Naphthalene (2a); Typical Procedure for Nickel-Catalyzed Reductive Alkylation of Aryl Sulfonates with Alkyl Iodides
General procedure: To a suspension of NiBr2·glyme (3.1 mg, 10 μmol), bathophenanthroline (3.9 mg, 12 μmol), manganese (22 mg, 0.40 mmol), and LiBr (26mg, 0.30 mmol) in DMF (0.4 mL) were added 2-naphthyl nonaflate (3a; 85.3 mg, 0.200 mmol) and 1-iodobutane (34.2 μL, 0.300 mmol) at r.t. After stirring for 14 h at 40 °C, the mixture was cooled to r.t. and to this was added aqueous phosphate buffer (pH 7.4, ca. 2 mL). The mixturewas extracted with Et2O (3 × ca. 2 mL) and the combined organic extracts were dried (Na2SO4) and filtered. The filtrate was concentrated under reduced pressure. The residue was purified by chromatography (silica gel, n-hexane) to give 2a
References:
Sumida, Yuto;Sumida, Tomoe;Hosoya, Takamitsu [Synthesis,2017,vol. 49,# 16,p. 3590 - 3601]
![2-Propenoic acid, 2-[[(1,1-dimethylethoxy)carbonyl]amino]-3-(2-naphthalenyl)-, methyl ester, (2Z)-](/CAS/20210305/GIF/176794-85-7.gif)
176794-85-7
0 suppliers
inquiry

176896-73-4
8 suppliers
inquiry
580-13-2
494 suppliers
$6.00/1g
93267-04-0
246 suppliers
$5.00/250mg

176896-73-4
8 suppliers
inquiry