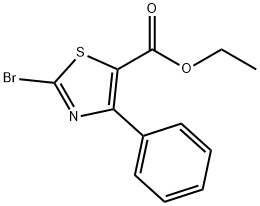
2-BROMO-4-PHENYL-5-THIAZOLECARBOXYLIC ACID ETHYL ESTER synthesis
- Product Name:2-BROMO-4-PHENYL-5-THIAZOLECARBOXYLIC ACID ETHYL ESTER
- CAS Number:177328-30-2
- Molecular formula:C12H10BrNO2S
- Molecular Weight:312.18

64399-23-1
102 suppliers
$26.00/250mg

177328-30-2
8 suppliers
inquiry
Yield:177328-30-2 98%
Reaction Conditions:
with tert.-butylnitrite;copper(I) bromide in acetonitrile at 0 - 20; for 16 h;Inert atmosphere;
Steps:
55.B Step B: Preparation of ethyl 2-bromo-4-phenylthiazole-5-carboxylate
1006661 Step B: Preparation of ethyl 2-bromo-4-phenylthiazole-5-carboxylate: A suspension of copper bromide (3.34 g, 14.9 mmol) in anhydrous acetonitrile (50 mL) was degassed with N2 for 10 minutes and cooled to 0 °C, then treated with t-butyl nitrite (2.2 mL, 18.7 mmol). This mixture was treated with a suspension of ethyl 2-amino-4-phenylthiazole-5- carboxylate (3.09 g, 12.4 mmol) in acetonitrile (50 mL) and the dark mixture was allowed to warm slowly to ambient temperature over 16 hours. The mixture was concentrated and the residue was dissolved in EtOAc (100 mL) and washed with NaHCO3 (100 mL). The aqueous layer was extracted with EtOAc (2 x 50 mL), and the combined organic phases were washed with water and brine, dried over Na2SO4, filtered and concentrated to afford ethyl 2-bromo-4- phenylthiazole-5-carboxylate (3.8 g, 98% yield) as a brown, crystalline solid. MS (apci) mlz 3 12.0 (M+).
References:
WO2014/78378,2014,A1 Location in patent:Paragraph 00666
55919-47-6
57 suppliers
$45.00/50mg

177328-30-2
8 suppliers
inquiry