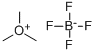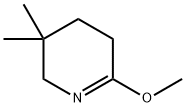
Pyridine, 2,3,4,5-tetrahydro-6-methoxy-3,3-dimethyl- synthesis
- Product Name:Pyridine, 2,3,4,5-tetrahydro-6-methoxy-3,3-dimethyl-
- CAS Number:179685-59-7
- Molecular formula:C8H15NO
- Molecular Weight:141.21
4007-79-8
20 suppliers
inquiry

179685-59-7
4 suppliers
inquiry
Yield:179685-59-7 82%
Reaction Conditions:
with trimethoxonium tetrafluoroborate in dichloromethane at 20; for 14 h;
Steps:
29.2
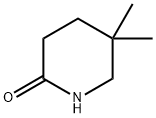
Trimethyloxonium tetrafluoroborate (97%, 11.9 g, 78 mmol) was added to the dry dichloromethane (156 mL) solution of 5,5-dimethyl-2-piperidone (9.93 g, 78 mmol) at room temperature and stirred for 14 h. The reaction mixture was neutralized with 10% sodium hydrogen carbonate aqueous solution, and the organic layer was separated. The aqueous layer was extracted with ethyl acetate (3×120 mL), then the combined organic layer was washed with 10% sodium hydrogen carbonate aqueous solution and brine. The organic layer was dried (MgSO4) and filtered. The filtrate was concentrated under reduced pressure and the titled compound was obtained as pale yellow oil (9.0 g, 82.0%). 1H NMR (CDCl3) δ 0.92 (s, 6H), 1.49 (t, 2H, J=7.0 Hz), 2.18 (t, 2H, J=7.0 Hz), 3.19 (s, 2H), 3.63 (s, 3H).
References:
US2006/276445,2006,A1 Location in patent:Page/Page column 37