
tert-butyl 4-(4-bromophenoxymethyl)piperidine-1-carboxylate synthesis
- Product Name:tert-butyl 4-(4-bromophenoxymethyl)piperidine-1-carboxylate
- CAS Number:180847-23-8
- Molecular formula:C17H24BrNO3
- Molecular Weight:370.28
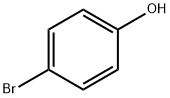
106-41-2
499 suppliers
$13.00/5g

161975-39-9
127 suppliers
$12.00/250mg

180847-23-8
6 suppliers
inquiry
Yield:180847-23-8 94%
Reaction Conditions:
with caesium carbonate in acetonitrile at 65; for 5 h;
Steps:
1.3 Step 3. Synthesis of tert-butyl 4-((4-bromophenoxy)methyl)piperidin-l-carboxylate:
tert-Butyl 4-((methylsulfonyloxy)methyl)piperidin-l-carboxylate (6.60 g, 22.49 mmol), 4-bromophenol (3.89 g, 22.49 mmol) and Cs2C03 (10.99 g, 33.74 mmol) were dissolved in acetonitrile (50 mL) at 65 C. The solution was stirred at the same temperature for 5 h. To the reaction mixture, saturated NH4C1 aqueous solution was added, and the mixture was extracted with ethyl acetate. The organic layer was washed with water, dried with anhydrous MgS04, and then concentrated under reduced pressure. The concentrate was purified by column chromatography (Si02, ethyl acetate / hexane = 5 % to 10 %), and concentrated to obtain the desired compound (7.88 g, 94%) as yellow oil .
References:
WO2015/80446,2015,A1 Location in patent:Page/Page column 21; 22
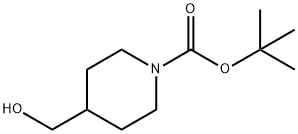
123855-51-6
425 suppliers
$6.00/5g

180847-23-8
6 suppliers
inquiry

24424-99-5
863 suppliers
$13.50/25G

180847-23-8
6 suppliers
inquiry
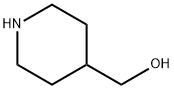
6457-49-4
391 suppliers
$18.00/25g

180847-23-8
6 suppliers
inquiry