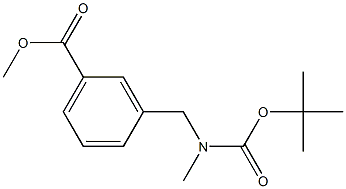
Methyl 3-(((tert-butoxycarbonyl)(methyl)amino)methyl)benzoate synthesis
- Product Name:Methyl 3-(((tert-butoxycarbonyl)(methyl)amino)methyl)benzoate
- CAS Number:180863-35-8
- Molecular formula:C15H21NO4
- Molecular Weight:279.3315
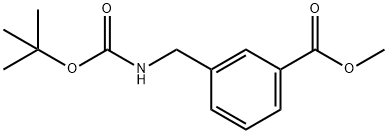
180863-55-2
40 suppliers
$12.00/100mg

74-88-4
344 suppliers
$15.00/10g

180863-35-8
4 suppliers
inquiry
Yield:180863-35-8 57%
Reaction Conditions:
Stage #1: methyl 3-(((tert-butoxycarbonyl)amino)methyl)benzoatewith sodium hydride in N,N-dimethyl-formamide;oil; for 1 h;
Stage #2: methyl iodide in N,N-dimethyl-formamide;oil; for 1 h;
Steps:
111 3-[(tert-Butoxycarbonyl-methyl-amino)-methyl]-benzoic acid methyl ester
Preparation 111 3-[(tert-Butoxycarbonyl-methyl-amino)-methyl]-benzoic acid methyl ester Dissolve 3-(tert-butoxycarbonylamino-methyl)-benzoic acid methyl ester (2.80 g, 10.6 mmol) in dimethylformamide (60 mL). Add 60% sodium hydride dispersion in oil (0.52 g, 13 mmol). Stir for 1 hr. Add methyl iodide (0.81 mL, 13 mmol) and stir for additional 1 hr. Quench the reaction with water and concentrate. Partition the residue between ethyl acetate and water. Separate the organic layer and dry over sodium sulfate. Filter and concentrate. Purify the residue by chromatography (silica gel) eluding with 15-25% ethyl acetate/hexane to provide the title compound (1.70 g, 57%).
References:
US2010/16373,2010,A1 Location in patent:Page/Page column 16
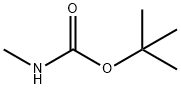
16066-84-5
127 suppliers
$10.00/1g
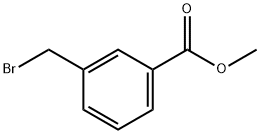
1129-28-8
240 suppliers
$13.43/1gm:

180863-35-8
4 suppliers
inquiry