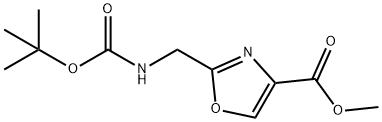
METHYL 2-((TERT-BUTOXYCARBONYLAMINO)METHYL)OXAZOLE-4-CARBOXYLATE synthesis
- Product Name:METHYL 2-((TERT-BUTOXYCARBONYLAMINO)METHYL)OXAZOLE-4-CARBOXYLATE
- CAS Number:182120-89-4
- Molecular formula:C11H16N2O5
- Molecular Weight:256.26
![4-Oxazolecarboxylic acid, 2-[[[(1,1-dimethylethoxy)carbonyl]amino]methyl]-4,5-dihydro-, methyl ester](/CAS/20210305/GIF/871715-69-4.gif)
871715-69-4
4 suppliers
inquiry

182120-89-4
21 suppliers
inquiry
Yield:182120-89-4 130 mg
Reaction Conditions:
with Bromotrichloromethane;1,8-diazabicyclo[5.4.0]undec-7-ene in dichloromethane at 0 - 25; for 12 h;Inert atmosphere;
Steps:
2 Step 3:
a solution of intermediate 3 (254 mg, 0.98 mmol) in DCM (24 mL) was added to BrCCb (0.7 mL, 2.94 mmol) at 0 °C. The mixture was stirred at 0 °C for 5 minutes before DBU (1.13 mL, 2.94 mmol) was added drop-wisely over 2 minutes. The mixture was stirred at 25 °C for 12 hours before quenched with ice-cooled aq.NaHCCh solution and extracted with DCM twice. The combined organic layers were washed with brine, dried over anhydrous Na2SC>4, filtered and concentrated to dryness. The residue was purified by chromatography on silica gel (PE: EtOAc= 3 : 1) to give intermediate 4 (130 mg, yield 60.7%) as yellow oil. LC/MS (ESI) (m/z): 257 (M+H)+.
References:
WO2020/198062,2020,A1 Location in patent:Page/Page column 336-338
![L-Serine, N-[(1,1-dimethylethoxy)carbonyl]glycyl-, methyl ester](/CAS/20210111/GIF/83661-73-8.gif)
83661-73-8
0 suppliers
inquiry

182120-89-4
21 suppliers
inquiry
![Serine, N-[(1,1-dimethylethoxy)carbonyl]glycyl-, methyl ester](/CAS/20210111/GIF/1038926-43-0.gif)
1038926-43-0
0 suppliers
inquiry

182120-89-4
21 suppliers
inquiry
![4-Oxazolecarboxylic acid, 2-[[[(1,1-dimethylethoxy)carbonyl]amino]methyl]-4,5-dihydro-, methyl ester, (4S)-](/CAS/20210305/GIF/182120-88-3.gif)
182120-88-3
0 suppliers
inquiry

182120-89-4
21 suppliers
inquiry
4530-20-5
531 suppliers
$5.00/5G

182120-89-4
21 suppliers
inquiry