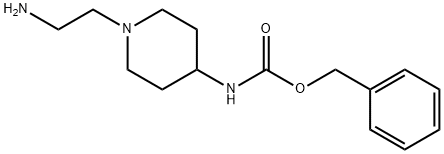
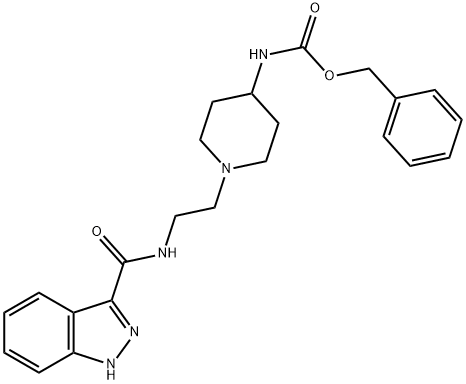
[1-(2-AMino-ethyl)-piperidin-4-yl]-carbaMic acid benzyl ester synthesis
- Product Name:[1-(2-AMino-ethyl)-piperidin-4-yl]-carbaMic acid benzyl ester
- CAS Number:182223-52-5
- Molecular formula:C15H23N3O2
- Molecular Weight:277.36
Yield:-
Reaction Conditions:
with sodium hydrogencarbonate in tetrahydrofuran;water;
Steps:
1 Preparation of 1-(2-aminoethyl)-4-benzyloxycarbonylaminopiperidine
Preparation 1 Preparation of 1-(2-aminoethyl)-4-benzyloxycarbonylaminopiperidine To a cold (10° C.) stirred mixture of 4-amino-1-benzyl-piperidine (15.2 g, 80 mmol), sodium bicarbonate (9.54 g, 110 mmol), 310 mL tetrahydrofuran and 155 mL water, was added dropwise benzyl chloroformate (14.0 mL, 98 mmol). The resulting mixture was stirred at 5°-10° C. for 2 h. The reaction mixture was poured onto 1000 mL water. Extraction with ethyl acetate, washing with brine, drying and evaporation of the ethyl acetate gave a viscous oil. Trituration with hexanes provided 18.56 g of solid. Mp 74°-76° C. Mass spectrum, m+ =324.
References:
US5654320,1997,A
![Carbamic acid, [1-[2-(1,3-dihydro-1,3-dioxo-2H-isoindol-2-yl)ethyl]-4-piperidinyl]-, phenylmethyl ester (9CI)](/CAS/20210305/GIF/182223-55-8.gif)
182223-55-8
0 suppliers
inquiry
![[1-(2-AMino-ethyl)-piperidin-4-yl]-carbaMic acid benzyl ester](/CAS/20120318/GIF/CB62577300.gif)
182223-52-5
5 suppliers
inquiry
50541-93-0
363 suppliers
$6.00/5g
![[1-(2-AMino-ethyl)-piperidin-4-yl]-carbaMic acid benzyl ester](/CAS/20120318/GIF/CB62577300.gif)
182223-52-5
5 suppliers
inquiry

182223-53-6
35 suppliers
$43.00/250mg
![[1-(2-AMino-ethyl)-piperidin-4-yl]-carbaMic acid benzyl ester](/CAS/20120318/GIF/CB62577300.gif)
182223-52-5
5 suppliers
inquiry
4498-67-3
465 suppliers
$6.00/5g
530-62-1
927 suppliers
$6.00/25g
![[1-(2-AMino-ethyl)-piperidin-4-yl]-carbaMic acid benzyl ester](/CAS/20120318/GIF/CB62577300.gif)
182223-52-5
5 suppliers
inquiry
182223-02-5
1 suppliers
inquiry
