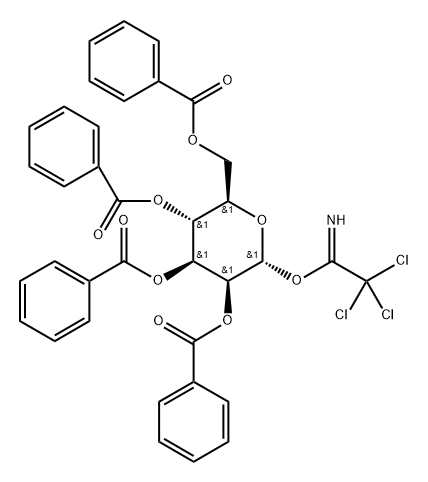
.alpha.-D-Mannopyranose, 2,3,4,6-tetrabenzoate 1-(2,2,2-trichloroethanimidate) synthesis
- Product Name:.alpha.-D-Mannopyranose, 2,3,4,6-tetrabenzoate 1-(2,2,2-trichloroethanimidate)
- CAS Number:183901-63-5
- Molecular formula:C36H28Cl3NO10
- Molecular Weight:740.97

7296-15-3
6 suppliers
inquiry

98-88-4
578 suppliers
$10.00/5g
545-06-2
231 suppliers
$10.00/5g

183901-63-5
27 suppliers
$155.00/500mg
Yield:183901-63-5 84%
Reaction Conditions:
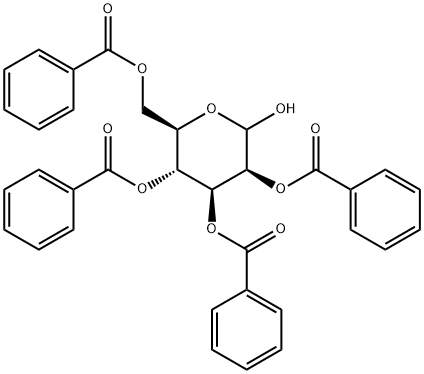
Stage #1: alpha-D-mannopyranoside;benzoyl chloridewith pyridine in toluene;Reflux;
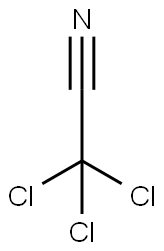
Stage #2: trichloroacetonitrilewith potassium carbonate in dichloromethane at 20;Inert atmosphere;
Steps:
100 ml of a three-necked flask, D-mannose (1.8 g, lOmol) was suspended in 25 mL of dry toluene and added with stirring (4.8 mL, 0.06 mol) was added dropwise a solution of BzCl (6.4 mL, 0.055 mol) in 20 mL of toluene over 15 minutes. To reflux, TLC (3: 1 petroleum ether-ethyl acetate) to test the reaction completely cooled to 0 ° C, filtered to remove pyridine hydrochloride, to the filter Liquid to the system by adding 100mL saturated methanol ammonia solution, stirring at room temperature reaction, timely monitoring to the end of the reaction. Insoluble, concentrated at low temperature to remove the solvent, vacuum drying 2 hours, under nitrogen protection plus 50mL anhydrous CH2C12, CC13CN 1.5mL (0.015 mol), K2C03 6.9 g (0.05 mmol), overnight at room temperature, TLC (3: 1 petroleum ether-ethyl acetate) The insoluble material was removed by suction filtration, concentrated and purified by column chromatography to give the compound of formula IV (6.2 g, yield 84%).
References:
CN105017346,2017,B Location in patent:Paragraph 0070-0072

545-06-2
231 suppliers
$10.00/5g
627466-98-2
29 suppliers
$110.00/1g

183901-63-5
27 suppliers
$155.00/500mg

96996-90-6
61 suppliers
$11.00/250mg

183901-63-5
27 suppliers
$155.00/500mg

7296-15-3
6 suppliers
inquiry

183901-63-5
27 suppliers
$155.00/500mg

98-88-4
578 suppliers
$10.00/5g

183901-63-5
27 suppliers
$155.00/500mg