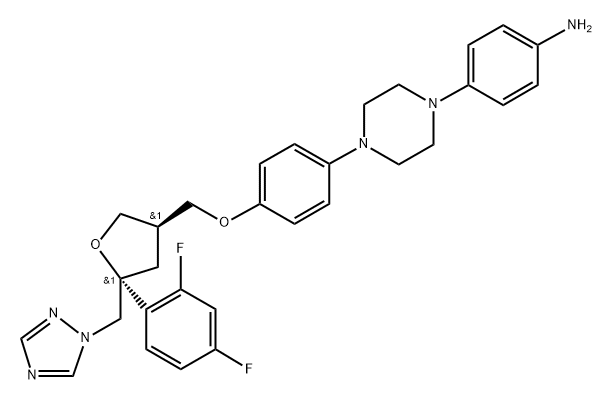
4-(4-{4-[(3R,5R)-5-(2,4-DIFLUORO-PHENYL)-5-[1,2,4]TRIAZOL-1-YLMETHYL-TETRAHYDRO-FURAN-3-YLMETHOXY]-PHENYL}-PIPERAZIN-1-YL)-PHENYLAMINE synthesis
- Product Name:4-(4-{4-[(3R,5R)-5-(2,4-DIFLUORO-PHENYL)-5-[1,2,4]TRIAZOL-1-YLMETHYL-TETRAHYDRO-FURAN-3-YLMETHOXY]-PHENYL}-PIPERAZIN-1-YL)-PHENYLAMINE
- CAS Number:184378-04-9
- Molecular formula:C30H32F2N6O2
- Molecular Weight:546.62
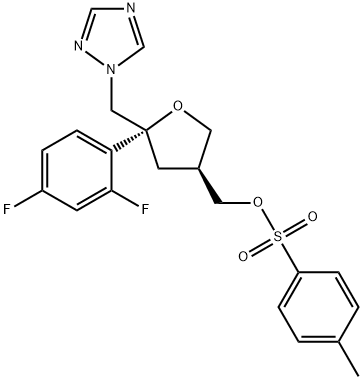
149809-43-8
376 suppliers
$56.00/100mg
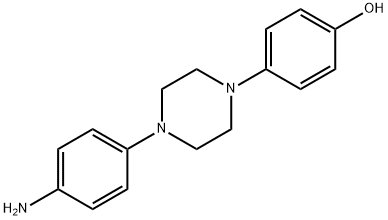
74853-08-0
255 suppliers
$81.00/5g
![4-(4-{4-[(3R,5R)-5-(2,4-DIFLUORO-PHENYL)-5-[1,2,4]TRIAZOL-1-YLMETHYL-TETRAHYDRO-FURAN-3-YLMETHOXY]-PHENYL}-PIPERAZIN-1-YL)-PHENYLAMINE](/CAS/20211123/GIF/184378-04-9.gif)
184378-04-9
10 suppliers
inquiry
Yield:184378-04-9 80%
Reaction Conditions:
Stage #1: 1-(4-aminophenyl)-4-(4-hydroxyphenyl)piperazinewith 2,6-di-tert-butyl-4-methyl-phenol;sodium hydroxide in water;dimethyl sulfoxide at 28 - 32; for 0.166667 h;Inert atmosphere;Large scale;
Stage #2: (2R-cis)-2-(2,4-difluorophenyl)-4-[[(4-methylphenyl)sulfonyloxy]methyl]-2-[(1H-1,2,4-triazol-1-yl)methyl]tetrahydrofuran in dimethyl sulfoxide at 28 - 32;Inert atmosphere;Large scale;
Steps:
1.2 Reference Example 1.2 Synthesis of the Compound of Formula (Ia)
A solution of 297 mg of BHT (butylated hydroxytoluene; M=220.35 g/mol; 1.35 mmol; 500 ppm) and 727 g of the compound of formula (A) [0417] (MW: 269.35 g/mol; 2.698 mol; 1.0 equiv.; obtainable, for example, according to example 1 of EP 1 230 231 B1 (on page 4)) in 4.7 l of DMSO was prepared at a mass temperature of 30±2° C. To this was added a solution (30±2° C.) of 161.9 g of NaOH (MW: 40.0 g/mol, 4.047 mol, 1.5 equiv.) in 161.9 g of oxygen-free H2O upon keeping the mass temperature at ≦32° C. The mixture was stirred for 10 min at 30±2° C. Then, a solution (30±2° C.) of 1335 g of the compound of formula (B) contained in the product as obtained according to Reference Example 1.1 (Reference Example 1.1.j) above (12.968 mol=1.1 equiv., MW=449.48 g/mol) in 6.6 l of DMSO was added at a mass temperature of ≦32° C. within 10 min. The pH of the reaction mixture was checked after a reaction time of 60 min and at least also after 5 and 8 hours. After stirring for 60 to 90 min at 30±2° C. the crystallization of the product started. The dark brownish, thin suspension was stirred for 10-15 h at 30±2° C. At minimum agitation rate, the addition of 21.8 l of H2O was started at 30±2° C. and was carried out at a constant rate whereby the reaction temperature was allowed to rise simultaneously to 45±5° C. Then, the remaining water was added at a rate to keep the temperature at 45±5° C. (overall addition time: about 60 min in total). Subsequently, the suspension was cooled to 20±2° C. in 60 min and stirred for further 60 min at 20±2° C. The resulting solid was filtered off and washed with 8.5 l of cold 1% oxygen-free aqueous NaOH (5-10° C.), then 2×8.5 l of cold oxygen-free H2O (15-10° C.) followed by 2×8.5 l of isopropanol (22±3° C.). [0419] All operations being part of the following purification procedure were carried out under a positive nitrogen atmosphere. [0420] The wet crude product (approx. 2.44 kg) in 72.7 l of acetonitrile was heated to reflux temperature. The mixture was refluxed for 10-15 minutes. To the resulting solution 116 g of charcoal (Ceca Eno) were added and the suspension was stirred for 10 min at reflux temperature. The charcoal was filtered off and the filter cake was washed with 11.6 l of hot acetonitrile. Under stirring the yellow coloured filtrate was cooled to 20±2° C. during 1 hour. Crystallization started at approx. 60° C. Under stirring the crystal suspension was cooled to 0±2° C. over 30 min and stirred at this temperature for one hour. The resulting crystals were filtered off and washed with 6 l of cold acetonitrile (≦5° C.). [0421] The product was dried at ≦75° C. (preferred temperature 70±5° C.) under reduced pressure (≦55 mbar) until a level of ≦1.4% of residual water was achieved. 1180 g of the compound of formula (Ia) (MW: 546.63 g/mol; 2.18 mol, 80.0% yield “as is”) were obtained as a white to yellow crystalline powder.
References:
US2014/303184,2014,A1 Location in patent:Paragraph 0416-0422
51336-94-8
313 suppliers
$6.00/5g
![4-(4-{4-[(3R,5R)-5-(2,4-DIFLUORO-PHENYL)-5-[1,2,4]TRIAZOL-1-YLMETHYL-TETRAHYDRO-FURAN-3-YLMETHOXY]-PHENYL}-PIPERAZIN-1-YL)-PHENYLAMINE](/CAS/20211123/GIF/184378-04-9.gif)
184378-04-9
10 suppliers
inquiry
![1,3-PROPANEDIOL, 2-[2-(2,4-DIFLUOROPHENYL)-2-PROPEN-1-YL]-](/CAS/GIF/165115-73-1.gif)
165115-73-1
87 suppliers
inquiry
![4-(4-{4-[(3R,5R)-5-(2,4-DIFLUORO-PHENYL)-5-[1,2,4]TRIAZOL-1-YLMETHYL-TETRAHYDRO-FURAN-3-YLMETHOXY]-PHENYL}-PIPERAZIN-1-YL)-PHENYLAMINE](/CAS/20211123/GIF/184378-04-9.gif)
184378-04-9
10 suppliers
inquiry
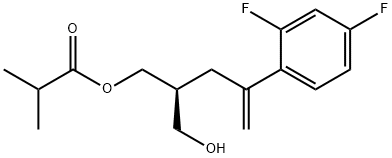
192448-07-0
22 suppliers
inquiry
![4-(4-{4-[(3R,5R)-5-(2,4-DIFLUORO-PHENYL)-5-[1,2,4]TRIAZOL-1-YLMETHYL-TETRAHYDRO-FURAN-3-YLMETHOXY]-PHENYL}-PIPERAZIN-1-YL)-PHENYLAMINE](/CAS/20211123/GIF/184378-04-9.gif)
184378-04-9
10 suppliers
inquiry
![Propanoic acid, 2-methyl-, [(3S)-5-(2,4-difluorophenyl)tetrahydro-5-(iodomethyl)-3-furanyl]methyl ester](/CAS/20200611/GIF/1350560-52-9.gif)
1350560-52-9
0 suppliers
inquiry
![4-(4-{4-[(3R,5R)-5-(2,4-DIFLUORO-PHENYL)-5-[1,2,4]TRIAZOL-1-YLMETHYL-TETRAHYDRO-FURAN-3-YLMETHOXY]-PHENYL}-PIPERAZIN-1-YL)-PHENYLAMINE](/CAS/20211123/GIF/184378-04-9.gif)
184378-04-9
10 suppliers
inquiry