
Tert-Butyl N-(3-[(Tert-Butyldimethylsilyl)Oxy]-2-Oxopropyl)Carbamate synthesis
- Product Name:Tert-Butyl N-(3-[(Tert-Butyldimethylsilyl)Oxy]-2-Oxopropyl)Carbamate
- CAS Number:184429-84-3
- Molecular formula:C14H29NO4Si
- Molecular Weight:303.47
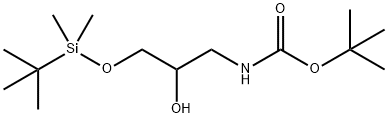
195197-94-5
![Tert-Butyl N-(3-[(Tert-Butyldimethylsilyl)Oxy]-2-Oxopropyl)Carbamate](/CAS/20180702/GIF/184429-84-3.gif)
184429-84-3
Oxalyl chloride (13.6 mL, 0.16 mol) was dissolved in anhydrous dichloromethane (150 mL) under nitrogen protection and cooled to -78 °C. Dimethyl sulfoxide (15.2 mL, 0.21 mol) was slowly added dropwise over 30 min, and after completion of the dropwise addition, stirring was continued for 1 h at -78 °C. Subsequently, a solution of tert-butyl 3-((tert-butyldimethylsilyl)oxy)-2-hydroxypropylcarbamate (32.6 g, 0.11 mol) in dichloromethane (50 mL) was added dropwise over 20 minutes. After the reaction mixture continued to be stirred at -78 °C for 1 h, triethylamine (59.6 mL, 0.43 mol) was added. The cooling bath was removed and the reaction mixture was allowed to slowly warm to room temperature. The reaction mixture was transferred to a partitioning funnel, water (100 mL) and dichloromethane (70 mL) were added for partitioning, and the aqueous layer was further extracted with dichloromethane (2 x 70 mL). The organic layers were combined, dried with anhydrous sodium sulfate and concentrated under nitrogen protection. The crude product was purified by silica gel column chromatography using 5% ethyl acetate in hexane solution as eluent to afford tert-butyl 3-((tert-butyldimethylsilyl)oxy)-2-oxopropylcarbamate (29.8 g, 92%) as a light yellow oil.1H-NMR (300 MHz, CDCl3) δ: 0.11 (6H, s), 0.94 (9H, s), and 1.47 (9H, s), 3.92 (2H, s), 4.26 (2H, d, J=4.6 Hz), 5.22 (1H, br s).

195197-94-5
26 suppliers
inquiry
![Tert-Butyl N-(3-[(Tert-Butyldimethylsilyl)Oxy]-2-Oxopropyl)Carbamate](/CAS/20180702/GIF/184429-84-3.gif)
184429-84-3
31 suppliers
$45.00/250mg
Yield: 92%
Reaction Conditions:
Stage #1:[3-(tert-butyldimethylsilanyloxy)-2-hydroxypropyl]carbamic acid tert-butyl ester with oxalyl dichloride;dimethyl sulfoxide in dichloromethane for 1.33333 h;Inert atmosphere;Cooling with ice;
Stage #2: with triethylamine in dichloromethane at 20;Inert atmosphere;
Steps:
1 Preparation of terf-butyl 3-(tert-butyldimethylsilyloxy)-2-oxopropylcarbamate
To a stirring solution of oxalyl chloride (13.6 mL, 0.16 mol) in dry CH2Cl2 (150 mL) at -78°C under N2 was added DMSO (15.2 mL, 0.21 mol) dropwise over 30 min. After complete addition the resulting solution was stirred at -78°C for 1 h. A solution of tert-butyl 3-(rt-butyldimethyl-silyloxy)-2-hydroxypropylcarbamate (32.6 g, 0.1 1 mol) in CH2Cl2 (50 mL) was then added dropwise over 20 min. Stirring was continued for a further 1 hour at which time triethylamine (59.6 mL, 0.43 mol) was added. The cooling bath was removed and the reaction mixture was allowed to warm to room temperature. The reaction mixture was partitioned between water (100 mL) and CH2Cl2 (70 mL) and the aqueous layer was extracted with further CH2Cl2 (2 x 70 mL); the combined organics were dried over Na2SO4 and concentrated under a stream of nitrogen gas. The crude residue was purified over silica gel eluting with 5% ethylacetate in n-hexane to give tert- butyl 3-(tert-butyldimethylsilyloxy)-2-oxopropylcarbamate (29.8 g, 92%) as a pale yellow oil. 1H-NMR (300 MHz; CDCl3) ppm: 0.11 (6 H, s), 0.94 (9 H, s), 1.47 (9 H, s), 3.92 (2 H, s), 4.26 (2 H, d, J4.6 Hz), 5.22 (1 H, br s).
References:
PHARMAXIS LTD.;DEODHAR, Mandar;FINDLAY, Alison, Dorothy;FOOT, Jonathan, Stuart;JAROLIMEK, Wolfgang;MCDONALD, Ian, Alexander;ROBERTSON, Alan;TURNER, Craig, Ivan WO2013/163675, 2013, A1 Location in patent:Paragraph 00182

24424-99-5
863 suppliers
$13.50/25G
![Tert-Butyl N-(3-[(Tert-Butyldimethylsilyl)Oxy]-2-Oxopropyl)Carbamate](/CAS/20180702/GIF/184429-84-3.gif)
184429-84-3
31 suppliers
$45.00/250mg
137618-48-5
89 suppliers
$6.00/250mg
![Tert-Butyl N-(3-[(Tert-Butyldimethylsilyl)Oxy]-2-Oxopropyl)Carbamate](/CAS/20180702/GIF/184429-84-3.gif)
184429-84-3
31 suppliers
$45.00/250mg
18162-48-6
670 suppliers
$9.00/5g
![Tert-Butyl N-(3-[(Tert-Butyldimethylsilyl)Oxy]-2-Oxopropyl)Carbamate](/CAS/20180702/GIF/184429-84-3.gif)
184429-84-3
31 suppliers
$45.00/250mg