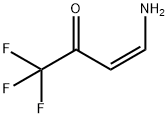
Methyl 3-Methoxy-3-((4,4,4-trifluoro-3-oxobut-1-en-1-yl)aMino)propanoate synthesis
- Product Name:Methyl 3-Methoxy-3-((4,4,4-trifluoro-3-oxobut-1-en-1-yl)aMino)propanoate
- CAS Number:186273-73-4
- Molecular formula:C9H12F3NO4
- Molecular Weight:255.1911
Yield:186273-73-4 88.3%
Reaction Conditions:
with sodium hydride in N,N-dimethyl-formamide at -15 - -10; for 3 h;Inert atmosphere;
Steps:
1
Under nitrogen protection, add 12.9g (323.5mmol) of NaH and 150g of DMF to the reaction flask at -15-10°C.4-amino-1,1,1-trifluoro-3-buten-2-one50 g (359.5 mmol) solution, the system turned from colorless to light yellow and finally to a red suspension,Methyl 3-methoxyacrylate (41.75 g, 359.5 mmol) was added dropwise and reacted at this temperature for 3 hours.Concentrated hydrochloric acid was added dropwise to the reaction flask at -10°C to adjust the pH of 8-9. The system changed from a deep red suspension to orange, and water was added.1.5L of dilute hydrochloric acid was used to adjust the pH to 1 to 2, and a large amount of white flocculent solids precipitated. After stirring for 0.5 hours, 81.5 g of a white product was obtained by filtration (yield, 88.3%).The NMR analysis of the product obtained in this example is shown in Figure 1.Prove that the resulting product isN-1-methoxy-2-methoxycarbonylethyl-4,4,4-trifluoro-3-keto-1-butenamine..
References:
CN107628991,2018,A Location in patent:Paragraph 0016; 0027; 0028; 0029; 0032; 0036