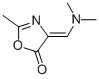
5(4H)-Oxazolone,4-[(dimethylamino)methylene]-2-methyl-(9CI) synthesis
- Product Name:5(4H)-Oxazolone,4-[(dimethylamino)methylene]-2-methyl-(9CI)
- CAS Number:188663-05-0
- Molecular formula:C7H10N2O2
- Molecular Weight:154.168
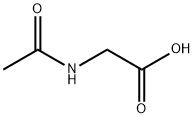
543-24-8
504 suppliers
$9.00/100g
![5(4H)-Oxazolone,4-[(dimethylamino)methylene]-2-methyl-(9CI)](/CAS/GIF/188663-05-0.gif)
188663-05-0
2 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1: N-acetylglycinewith N,N-dimethyl-formamide;trichlorophosphate at 0 - 45; for 1.5 h;
Stage #2: with ammonia in water at 0 - 10; for 1 h;
Steps:
R.1
EXAMPLES Reference Example 1. 4-(N,N-dimethylaminomethylene)-2-methyl-5-oxazolinone. Phosphorus oxychloride (67.0 g, 437 mmol) and N,N-dimethylformamide (33.0 g, 451 mmol) were added to N-acetylglycine (20.0 g, 171 mmol) in an ice bath, and the mixture was stirred at 45°C for 1.5 hours. The reaction mixture was concentrated under reduced pressure and added dropwise to 28% aqueous ammonia (150 ml) while maintaining the temperature at not higher than 10°C. The reaction mixture was stirred for 1 hour in an ice-bath and the precipitate was collected by filtration. The obtained crystals were washed successively with water and ethanol and dried to give the title compound as crystals (20.2 g, 131 mmol). 1H-NMR(CDCl3)δ: 2.21(3H, s), 3.18(3H, m), 3.47(3H, s), 6.96(1H, s). MS(ESI) m/z [MH]+ 155.2
References:
EP1577304,2005,A1 Location in patent:Page/Page column 10