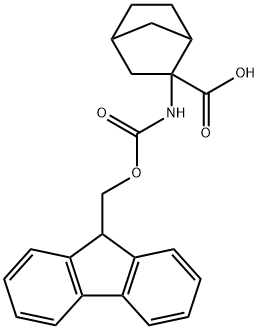
Fmoc-2-aminobicyclo[2.2.1]heptane-2-carboxylic acid (mixture of isomers) synthesis
- Product Name:Fmoc-2-aminobicyclo[2.2.1]heptane-2-carboxylic acid (mixture of isomers)
- CAS Number:188751-57-7
- Molecular formula:C23H23NO4
- Molecular Weight:377.43
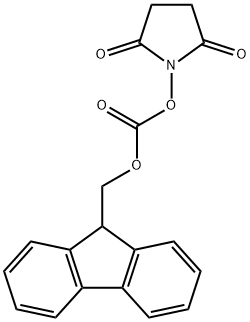
82911-69-1
606 suppliers
$7.00/25g
20448-79-7
73 suppliers
$29.00/25mg
![Fmoc-2-aminobicyclo[2.2.1]heptane-2-carboxylic acid (mixture of isomers)](/CAS/20140525/GIF/CB3974137.gif)
188751-57-7
3 suppliers
inquiry
Yield:188751-57-7 21%
Reaction Conditions:
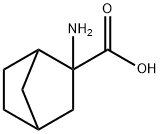
Stage #1: 2-aminonorbornane-2-carboxylic acidwith hydrogenchloride in water; pH=10;
Stage #2: N-(9H-fluoren-2-ylmethoxycarbonyloxy)succinimide in water;acetonitrile at 20;
Steps:
A
2-(((9H-Fluoren-9-yl)methoxy)carbonylamino)bicyclo[2.2.1]heptane-2-carboxylic acid (compound 11 of example A): After the solution of compound 9 of example A was adjusted to pH= 10 with 2 N HCI, Fmoc-Osu (15.87 g, 45.0 mmol) and acetonitrile (50.0 mL) were added. The reaction mixture was stirred at room temperature overnight. Most of the solvent was removed under reduced pressure and the resulting mixture was adjusted to pH = 2 with 2 N HCI and extracted with DCM. The combined extracts were washed with brine, dried over anhydrous Na2S04 and concentrated. The residue was purified by flash column chromatography on silica gel (DCM/MeOH = 50: 1) to afford compound 11 of example A (9.0 g, 21% yield over three steps) as a white solid.
References:
WO2012/119941,2012,A1 Location in patent:Page/Page column 111
497-38-1
163 suppliers
$13.00/1g
![Fmoc-2-aminobicyclo[2.2.1]heptane-2-carboxylic acid (mixture of isomers)](/CAS/20140525/GIF/CB3974137.gif)
188751-57-7
3 suppliers
inquiry
![spiro[imidazolidine-5,2-norbornane]-2,4-dione](/CAS/20180529/GIF/22264-49-9.gif)
22264-49-9
6 suppliers
inquiry
![Fmoc-2-aminobicyclo[2.2.1]heptane-2-carboxylic acid (mixture of isomers)](/CAS/20140525/GIF/CB3974137.gif)
188751-57-7
3 suppliers
inquiry