D-arabino-Hex-1-enitol, 1,5-anhydro-4,6-O-[bis(1,1-dimethylethyl)silylene]-2-deoxy- synthesis
- Product Name:D-arabino-Hex-1-enitol, 1,5-anhydro-4,6-O-[bis(1,1-dimethylethyl)silylene]-2-deoxy-
- CAS Number:191593-14-3
- Molecular formula:C14H26O4Si
- Molecular Weight:286.44
Yield:191593-14-3 91%
Reaction Conditions:
Stage #1: D-glucal triacetatewith potassium carbonate in methanol at 20; for 12 h;
Stage #2: di-tert-butylsilyl bis(trifluoromethanesulfonate)with pyridine in N,N-dimethyl-formamide at -40 - 20; for 1 h;
Steps:
Synthesis of (4aR,8R,8aS)-2,2-di-tert-butyl-4,4a,8,8a-tetrahydropyrano[3,2-d][1,3,2]dioxasilin-8-ol (1)
To a solution of (+)-Tri-0-acetyl-D-glucal (14g, 51.4 mmol) in MeOH (50 ml_) was added K2CO3 (100 mg) and the mixture was stirred for 12 h at room temperature. Then, MeOH was evaporated under reduced pressure and the resultant residue was dissolved in CHCI3 and concentrated again for three times. The resulting solid was dissolved in DMF (40 ml), pyridine (20 ml_, 257.1 mmol) was added and cooled to - 40°C. Then, terc- Bu2Si(OTf)2 (18.3 ml_, 56.6 mmol) was dropwise added and the mixture was stirred for 1 h till room temperature. After that time, EtOAc (30 ml_) was added and the organic layer washed with a 15% aqueous solution of CUSO4 (2x30 ml_), water (3x30 ml_) and dried over Na2SC>4, filtered and the solvent evaporated under reduced pressure. The residue was purified by column chromatography with ethyl acetate-hexane (5%) to give compound 1 (13.4 g, 91 %) as a white solid (Mp: 84-85°C), Rf: 0.71 (EtOAc). IR (ATR, cm-1): 3440, 2988, 2285, 1646, 1215, 869.1H-NMR (CDCI3, d): 6.25 (1 H, dd, J=6.1/1.8 Hz, H-1), 4.74 (1 H, dd, J=6.1/1.9 Hz, H-2), 4.25 (1 H, m, H- 3), 4.16 (1 H, dd, J=10.2/4.9 Hz, H-6), 3.93 (2H, m, H-6, H-4), 3.82 (1 H, m, H-5), 2.66(1 H, s, OH); 1.05 (9H, s, CH3-u), 0.98 (9H, s, CH3-u) ppm.1 3C-NMR (CDCI3, d): 143.5 (CH-1), 103.1 (CH-2), 77.3 (CH-4), 72.2 (CH-5), 70.0 (CH-3), 65.6 (CH2-6), 27.4 (CH3-U), 26.8 (CH3-U), 22.6 (C-u), 19.7 (C-u) ppm. MS(ESI) [m/z, (%)]: 287 (M++1 , 7), 286 (M+, 12), 269 (M+-OH, 100). HRMS (ESI): 286.1232 calculated for C1 4H2604Si;found 286.1647.
References:
WO2020/229470,2020,A1 Location in patent:Page/Page column 30-31

85272-31-7
164 suppliers
$25.00/1g
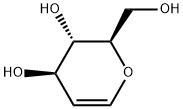
13265-84-4
226 suppliers
$14.00/1g
191593-14-3
0 suppliers
inquiry
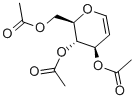
2873-29-2
367 suppliers
$5.00/1g
191593-14-3
0 suppliers
inquiry
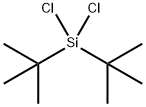
18395-90-9
78 suppliers
$110.00/1g

13265-84-4
226 suppliers
$14.00/1g
191593-14-3
0 suppliers
inquiry