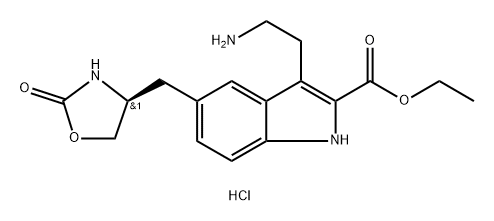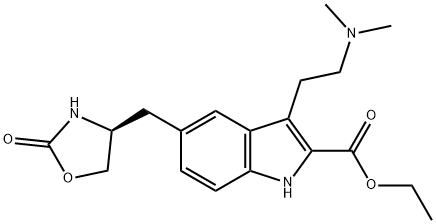
Zolmitriptan Related Compound D (20 mg) ((S)-Ethyl 3-[2-(dimethylamino)ethyl]-5-[(2-oxooxazolidin-4-yl)methyl]-1H-indole-2-carboxylate) synthesis
- Product Name:Zolmitriptan Related Compound D (20 mg) ((S)-Ethyl 3-[2-(dimethylamino)ethyl]-5-[(2-oxooxazolidin-4-yl)methyl]-1H-indole-2-carboxylate)
- CAS Number:191864-24-1
- Molecular formula:C19H25N3O4
- Molecular Weight:359.42
Yield:191864-24-1 4.6 kg
Reaction Conditions:
Stage #1: ethyl (S)-3-(2-aminoethyl)-5-((2-oxooxazolidin-4-yl)methyl)-1H-indole-2-carboxylate hydrochloridewith sodium cyanoborohydride;acetic acid in methanol at 10 - 15;Industrial scale;
Stage #2: formaldehyd in methanol;water at 0;Industrial scale;
Steps:
Preparation of 4-(S)-((3-(2-(Dimethylamino)ethyl)-1H-indol-5-yl)methyl)-oxazolidin-2-one 1
To a stirred solution of (S)-ethyl-3-(2-aminoethyl)-5-((2-oxooxazolan-4-yl)methyl]-1H-indole-2-carboxylate hydrochloride 8 (5.0 kg, 13.6 mol) in methanol (50 L) at room temperature, 20% sodium carbonate solution was added dropwise up to pH 7. Glacial acetic acid (1.94 L, 2.04 kg, 34.01 mol) was added to the neutralized solution at the same temperature. The reaction mass was cooled to 10-15 °C, and sodium cyanoborohydride (1.51 kg, 24.48 mol) was added pinch by pinch to the reaction mass under stirring at 10-15 °C. After the addition, the reaction mass was further cooled to °0 C, and a mixture of aqueous formaldehyde solution (38%, 3.7 L, 45.5 mol) and methanol (15 L) was added dropwise to the stirred reaction mass. The reaction mass was stirred at the same temperature for another 60-90 min. After the completion of the reaction (via TLC, using 30% methanol and 1.5% NH3 in dichloromethane as the mobile phase), an aqueous solution of sodium carbonate [15L of 20% (w/v)] was added at 0 °C, and the reaction mass was extracted twice with dichloromethane (250 L). Combined organic layers were washed with DM water (25 L) followed by brine (25 L), and removal of solvent furnished the crude ethyl 3-(2-dimethylaminoethyl)-5-[(4S)-2-oxo-oxazolidin-4-ylmethyl]-1H-indole-2-carboxylate 9 in 4.6 kg yield. Aqueous solution of sodium carbonate (1.36 kg, 12.8mol in 46L water) was added to the crude compound and the stirred reaction mass was refluxed for 2-2.5 h. After the completion of the reaction (via TLC, using 30% methanol in dichloromethane as the mobile phase), the crude reaction mass was allowed to cool to 15-18 °C, and concentrated hydrochloric acid (35%, 13.8L, 3.0 vol. with regard to compound 9) was added dropwise to the reaction mass. The reaction mixture was again refluxed for 4 h, and after completion of the reaction (via TLC, using 30% MeOH and 1.5% NH3 in dichloromethane; the reaction mixture was cooled to 20-25 °C and washed with 50L of ethyl acetate. The aqueous layer was further cooled to 15-20 °C, basified with 20% sodium hydroxide solution up to pH 9.5-10 and extracted three times with ethyl acetate (392 L) at 25-30 °C. Combined ethyl acetate layers were washed twice with water (29.2 L) and evaporated to dryness to yield 2.5 kg of solid residue. Isopropyl alcohol (7.5 L) was added to the solid residue and slowly heated to reflux, resulting in a clear solution. The solution was slowly cooled to 20-30 °C. N-heptane (2.5 L) was added dropwise to it, stirred for 15-20 min, and then filtered. The solid residue was washed with prechilled 50:50 mixture (2.5 L) of isopropanol and n-heptane and finally washed with n-heptane (5.0 L) and dried at 50-55 °C under 50-100 mm vacuum to obtain the title compound 1 in an overall yield of 1.75 kg (45%); mp: 138-139 °C; purity: 98.55%; 1H NMR (CDCl3, 500MHz): δ 8.0 (br s, 1H), 7.4 (s,1H), 7.35 (d, 1H, J=8.5 Hz), 7.05 (s, 1H), 6.95 (d, 1H, J=8.5 Hz), 5.0 (s, 1H), 4.5 (t,1H, J=8.0 Hz), 4.2 (q, 1H, J=5.5 Hz and 8.5 Hz), 4.1 (q, 1H, J=5.5Hz and 11.5 Hz), 3.05-2.90 (m, 4H), 2.62 (t, 2H, 8.0Hz), 2.3 (s, 6H); 13C NMR (DMSO-d6, 50MHz): δ 23.1, 40.7, 45.1, 53.2, 60.0, 68.1, 111.3, 112.5, 118.8, 122.5, 122.7, 125.1, 127.6, 135.2, 158.8; IR νmax cm-1(KBr): 3240, 2920, 2854, 2772, 1744, 1480, 1441, 1405, 1241, 1070, 1031 cm-1; MS (electronspray): m/z 288.1 (M+1)+.
References:
Vujjini, Satish Kumar;Mothukuri, Vivekananda Reddy;Islam, Aminul;Bandichhor, Rakeshwar;Kagga, Mukkanti;Malakondaiah, Golla China [Synthetic Communications,2013,vol. 43,# 24,p. 3294 - 3306]
89288-22-2
22 suppliers
$165.00/0.25g
![Zolmitriptan Related Compound D (20 mg) ((S)-Ethyl 3-[2-(dimethylamino)ethyl]-5-[(2-oxooxazolidin-4-yl)methyl]-1H-indole-2-carboxylate)](/CAS/20150408/GIF/191864-24-1.gif)
191864-24-1
34 suppliers
inquiry
17193-40-7
202 suppliers
$14.00/1g
![Zolmitriptan Related Compound D (20 mg) ((S)-Ethyl 3-[2-(dimethylamino)ethyl]-5-[(2-oxooxazolidin-4-yl)methyl]-1H-indole-2-carboxylate)](/CAS/20150408/GIF/191864-24-1.gif)
191864-24-1
34 suppliers
inquiry
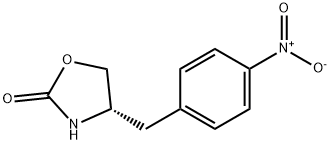
139264-55-4
49 suppliers
inquiry
![Zolmitriptan Related Compound D (20 mg) ((S)-Ethyl 3-[2-(dimethylamino)ethyl]-5-[(2-oxooxazolidin-4-yl)methyl]-1H-indole-2-carboxylate)](/CAS/20150408/GIF/191864-24-1.gif)
191864-24-1
34 suppliers
inquiry

69472-61-3
1 suppliers
inquiry
![Zolmitriptan Related Compound D (20 mg) ((S)-Ethyl 3-[2-(dimethylamino)ethyl]-5-[(2-oxooxazolidin-4-yl)methyl]-1H-indole-2-carboxylate)](/CAS/20150408/GIF/191864-24-1.gif)
191864-24-1
34 suppliers
inquiry