
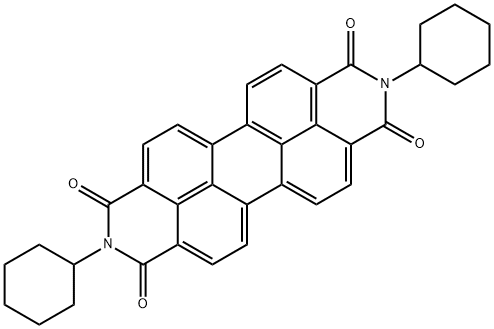
1,7-dibromo-N,N'-dicyclohexyl-perylene-3,4,9,10-tetracarboxylic acid diimide synthesis
- Product Name:1,7-dibromo-N,N'-dicyclohexyl-perylene-3,4,9,10-tetracarboxylic acid diimide
- CAS Number:191997-95-2
- Molecular formula:C36H28Br2N2O4
- Molecular Weight:712.43
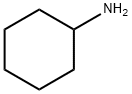
108-91-8
589 suppliers
$10.00/5mL

118129-60-5
104 suppliers
$27.00/250mg

191997-95-2
2 suppliers
inquiry
Yield:191997-95-2 77%
Reaction Conditions:
in 1-methyl-pyrrolidin-2-one at 85;
Steps:
1
Commercially available Perylene-3,4.9,10-tetracarboxylιc dianhyd?de (100.0 g, 0.255 mol) was brominated with mixture of bromine (29 rmL) and Iodine (2.38 g) in 100% sulfuric acid (845 mL) at.~ 85° C The yield of 1 ,7-dibromoperylene-3,4-9,10-tetracarboxylic dianhydride was 90 g (64%)Analysis: calculated' C24H6Br2O6, C 52.40, H 1.10, Br 29 05, O 17.45 %; found: C 52 29, H, 1.07, Br 28, 79 %. Absorption spectrum (9.82x105 M solution in 93% sulfuric acid)- 405 (9572), 516 (27892), 553 (37769).N.N'-Dicyclohexyl-i y-dibromopeiylene-SAθ.IO-tetracarboxydiimide was synthesized by reaction of 1 ,7-dιbromoperylene-3,4.9,10-tetracarboxylιc dianhydride (30.0 g) with cyclohexylamine (18.6 mL) in N-methylpyrrolidone (390 mL) at -85 ° C. The yield of N,N'-dιcyclohexyl-1 ,7- dibromoperylene-3,4:9,10-tetracarboxydiimide was 30 g (77%).N.N'-Dicyclohexyl-I J-di^ct-i-ynyOperylene-S^ θ.IO-tetracarboxydiimide by Sonogashira reaction: N.N'-dicyclohexyl-I J-dibromperylene-S^ θ.IO-tetracarboxydiimide (24.7 g) and octyne-1 (15 2 g) in the presence of bis(triphenylphosphine)palladium(ll) chloride (2.42 g), triphenylphospine (0 9 g),and copper(l) iodide (0.66 g). The yield of N.N'-dicyclohexyl-i y-dKoct-i-ynyOperylene-SAθ.IO- tetracarboxydiimide was 15.7 g (60 %).N,N'-Dicyclohexyl-5,11-dιhexylcoronene-2,3:8,9-tetracarboxydiimιde was synthesized by heating of N,N'-dicyclohexyl-1 ,7-dι(oct-1-ynyl)perylene-3,4.9,10-tetracarboxydιιmide (7.7 g) in toluene (400 mL) in the presence of 1 ,8-Diazabicyclo[5 4.0]undec-7-ene (0 6 ml) at 100-110° C for 20 hours.5,1 i-dihexylcoronene^S'δ.θ-tetracarboxylic dianhydride was prepared by hydrolysis of N1N'- dicyclohexyl-5,11-dihexylcoronene-2,3:8,9-tetracarboxydiimide (6 4 g, 8 3 mmol) with Potassium
References:
WO2009/109782,2009,A2 Location in patent:Page/Page column 12-13
41572-86-5
13 suppliers
inquiry

191997-95-2
2 suppliers
inquiry

128-69-8
289 suppliers
$6.00/5g

191997-95-2
2 suppliers
inquiry
1080663-33-7
1 suppliers
inquiry

191997-95-2
2 suppliers
inquiry