
CarbaMic acid,[trans-4-[[[(1,1-diMethylethoxy)carbonyl]aMino]Methyl]cyclohexyl]-, phenylMethyl ester synthesis
- Product Name:CarbaMic acid,[trans-4-[[[(1,1-diMethylethoxy)carbonyl]aMino]Methyl]cyclohexyl]-, phenylMethyl ester
- CAS Number:192323-61-8
- Molecular formula:C20H30N2O4
- Molecular Weight:362.46
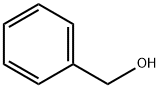
100-51-6
1415 suppliers
$5.00/100g
![CarbaMic acid,[trans-4-[[[(1,1-diMethylethoxy)carbonyl]aMino]Methyl]cyclohexyl]-, phenylMethyl ester](/CAS/20150408/GIF/192323-61-8.gif)
192323-61-8
8 suppliers
inquiry
Yield:192323-61-8 79%
Reaction Conditions:
Stage #1: trans 4-(tert-butyloxycarbonylamino)methylcyclohexanecarboxylic acidwith diphenyl phosphoryl azide;triethylamine in toluene at 25 - 80; for 4 h;
Stage #2: benzyl alcohol in toluene at 80 - 120;
Steps:
257
KOH (14 g) and Boc2O (33.3 g) were added to a solution of trans-4-(Aminomethyl)cyclohexane-carboxylic acid (20 g) in dioxane (112 mL) at 0° C. The reaction was stirred at 25° C. overnight. The solution was concentrated to half of the original volume under vacuum, acidified with 2.5 N HCl (PH=3), and extracted with EtOAc. The combined organic layer was washed with brine, dried over anhydrous MgSO4, filtered, and concentrated to give a white solid Intermediate 257-I (31.9 g). To a suspension of the above solid in toluene (150 mL) were added phosphorazidic acid diphenyl ester (32.4 g) and Et3N (11.9 g) at 25° C. for 1.0 hour. The reaction mixture was warmed to 80° C. for 3.0 hours and then cooled to 25° C. After benzyl alcohol (20 g) was added, the reaction mixture was stirred at 80° C. for another 3.0 hours and then warmed to 120° C. overnight. It was then concentrated and dissolved again in EtOAc and H2O. The organic layer was then collected. The aqueous layer was extracted with EtOAc. The combined organic layer was washed with 2.5 N HCl, saturated aqueous NaHCO3 and brine, dried over anhydrous MgSO4, filtered, and concentrated. The residue thus obtained was purified by column chromatography on silica gel (EtOAc/Hexane=1/2) to give Intermediate 257-II (35 g) in a 79% yield. To a suspension of Intermediate 257-II (1.9 g) in MeOH (10 mL) was added 10% Pd/C (190 mg). The mixture was stirred at ambient temperature under hydrogen atmosphere for 4.0 hours, filtered, and concentrated. The residue thus obtained was purified by column chromatography on silica gel (using EtOAc and MeOH as an eluant) to give Intermediate 257-III (750 mg) in a 60% yield. 222-III (1,198 mg) prepared from Example 222 was added to a solution of Intermediate 257-III (750 mg) in CH2Cl2 (30 mL). The mixture was stirred at 25° C. for 2 hours. NaBH(OAc)3 (1,046 mg) was then added at 25° C. for 12 hours. After the solution was concentrated, a saturated aqueous NaHCO3 solution was added to the resultant residue. The mixture was extracted with CH2Cl2. The organic layer was collected and concentrated. The residue thus obtained was purified by column chromatography on silica gel (using EtOAc and MeOH as an eluant) to afford Intermediate 257-IV (1,200 mg) in a 78% yield. A solution of Intermediate 257-IV (5.2 g) treated with 4 N HCl/dioxane (39 mL) in MeOH (52 mL) was stirred at room temperature for 8 hours. After ether (104 mL) was added, the solution was filtered. The solid thus obtained was dried under vacuum. K2CO3 (21 g) was added to a suspension of this solid in CH3CN (230 mL) at room temperature for 10 minutes. After water (9 mL) was added, the reaction mixture was stirred at room temperature for 2 hours. The mixture was then filtered, dried over anhydrous MgSO4, and concentrated to afford crude Intermediate 257-V (2.8 g). Crude Intermediate 257-V (2.8 g) and Et3N (1.3 mL) in 1-pentanol (11.3 mL) was allowed to react with 2,4-dichloro-6-aminopyrimidine (1,633 mg) at 100° C. for 12 hours. The solvent was then removed and the residue was purified by column chromatography on silica gel (21% NH3 (aq)/MeOH=1/19) to afford Intermediate 257-VI (3.3 g) in a 75% yield. A solution of Intermediate 252-VI (3.3 g) and Boc2O (4.189 g) in CH2Cl2 (60 mL) was added to Et3N (1.0 mL) at 25° C. overnight. The solution was then concentrated and the resultant residue was purified by column chromatography on silica gel (using EtOAc and Hexane as an eluant) to give Intermediate 257-VII (3.2 g) in a 64% yield. Intermediate 257-VII (2.6 g) and piperazine (1.127 g) in 1-pentanol (5.2 mL) was added to Et3N (0.5 mL) at 120° C. for 18 hours. After the solution was concentrated, the residue was treated with water and extracted with CH2Cl2. The organic layer was collected and concentrated. The residue thus obtained was purified by column chromatography on silica gel (using EtOAc/MeOH to 21% NH3 (aq)/MeOH as an eluant) to afford Intermediate 257-VIII (1.8 g) in a 64% yield. To a solution of Intermediate 257-VIII (200 mg) in CH3CN (20 mL) were added ethyl bromoacetate (52 mg) and K2CO3 (128 mg). The mixture was stirred at 60° C. for 2 hours. The solution was filtered and concentrated. The residue was purified by column chromatography on silica gel (using EtOAc and MeOH as an eluant) to afford Intermediate 257-IX (140 mg) in a 62% yield. 0.5 M of a LiOH aqueous solution (10 mL) was added to Intermediate 257-IX (500 mg) dissolved in THF (10 mL). The mixture was stirred at room temperature for 15 hours. It was then acidified with 2.5 M HCl (pH=9) and filtered to obtain a yellow solid. The yellow solid was purified by column chromatography on silica gel (using EtOAc/MeOH to 21% NH3 (aq)/MeOH as an eluant) to afford Intermediate 257-X (337 mg) in a 70% yield. 20% TFA/CH2Cl2 (10 mL) was added to a solution of Intermediate 257-X (400 mg) in CH2Cl2 (8 mL). The solution was stirred at room temperature for 2 hours and then concentrated. To the residue in acetone (7 mL) was added HCl (4 M in dioxane, 1.3 mL) at room temperature for 30 minutes. After the solvents were removed, the residue was treated with ether and filtered to give hydrochloride salt of Compound 257 (257 mg). CI-MS (M++1): 503.4.
References:
US2006/281712,2006,A1 Location in patent:Page/Page column 122-124
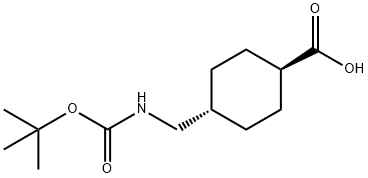
27687-14-5
111 suppliers
$10.00/1g

100-51-6
1415 suppliers
$5.00/100g
![CarbaMic acid,[trans-4-[[[(1,1-diMethylethoxy)carbonyl]aMino]Methyl]cyclohexyl]-, phenylMethyl ester](/CAS/20150408/GIF/192323-61-8.gif)
192323-61-8
8 suppliers
inquiry