
5H-1,4-Benzodiazepin-5-one, 2-chloro-7-fluoro-3,4-dihydro-4-methyl- synthesis
- Product Name:5H-1,4-Benzodiazepin-5-one, 2-chloro-7-fluoro-3,4-dihydro-4-methyl-
- CAS Number:193693-31-1
- Molecular formula:C10H8ClFN2O
- Molecular Weight:226.63

78755-80-3
78 suppliers
$115.00/10mg

193693-31-1
2 suppliers
inquiry
Yield:-
Reaction Conditions:
with N,N-dimethyl-aniline;trichlorophosphate in acetonitrile at 80 - 90; for 3 h;Large scale;
Steps:
1 Example 1 Preparation of Formula III
Add acetonitrile (15L) to the 20L reactor,Turn on the stirring,Adding formula II (1.0kg),N,N-dimethylaniline (5.8kg),Phosphorus oxychloride (1.1kg).The reaction solution was heated to 80-90°C and refluxed for 3 hours,After the reaction,Steam out acetonitrile,Then cool down to 10-20°C.Slowly add 10% sodium bicarbonate aqueous solution dropwise,The temperature is controlled at 20-30°C.After quenching is complete,Stir for 10-20 minutes,No bubbles are observed,Extract twice with dichloromethane (5L*2),The combined organic layer was washed once with 5 L of saturated brine,Concentrate and recover the dichloromethane to obtain the N,N-dimethylaniline solution of chloroimine,Go directly to the next step without purification.
References:
CN112457317,2021,A Location in patent:Paragraph 0039; 0040; 0041

79-37-8
462 suppliers
$17.67/10gm:

78755-80-3
78 suppliers
$115.00/10mg

193693-31-1
2 suppliers
inquiry
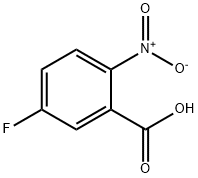
320-98-9
283 suppliers
$8.00/5g

193693-31-1
2 suppliers
inquiry
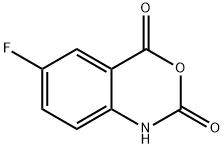
321-69-7
140 suppliers
$19.00/1g

193693-31-1
2 suppliers
inquiry