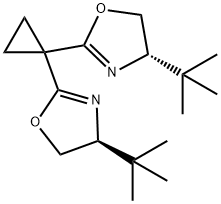
(4S,4'S)-2,2'-Cyclopropylidenebis[4-tert-butyl-4,5-dihydro oxazole],99%e.e. synthesis
- Product Name:(4S,4'S)-2,2'-Cyclopropylidenebis[4-tert-butyl-4,5-dihydro oxazole],99%e.e.
- CAS Number:195379-09-0
- Molecular formula:C17H28N2O2
- Molecular Weight:292.42
![1,1-Cyclopropanedicarboxamide, N,N'-bis[(1S)-1-(hydroxymethyl)-2,2-dimethylpropyl]-](/CAS/20210305/GIF/874916-76-4.gif)
874916-76-4
0 suppliers
inquiry
![(4S,4'S)-2,2'-Cyclopropylidenebis[4-tert-butyl-4,5-dihydro
oxazole],99%e.e.](/CAS/20180527/GIF/195379-09-0.gif)
195379-09-0
25 suppliers
inquiry
Yield:195379-09-0 77%
Reaction Conditions:
with dmap;methanesulfonyl chloride;triethylamine in dichloromethane at 0 - 60; for 2.16667 - 12.1667 h;Product distribution / selectivity;
Steps:
5; 7-3; 8-3
Example 5; In a 100 mL Schlenk tube purged with nitrogen, 490 mg (1.49 mmol) of N,N'-bis[(S)-1-tert-butyl-2-hydroxyethyl]cyclopropane-1,1-dicarboxamide, 664 mg (6.56 mmol) of triethylamine, 18.2 mg (0.15 mmol) of 4-dimethylaminopyridine and 30 mL of dichloromethane were mixed and a homogeneous solution was obtained. The solution was cooled to 0°C. After 345 mg (3.01 mmol) of methanesulfonyl chloride was added dropwise to the solution over 10 minutes at the same temperature, the resulting mixture was heated to 20°C and stirred for 12 hours. After a saturated aqueous ammonium chloride solution was added thereto and stirred for 10 minutes at room temperature, the separation was conducted to obtain the oil layer. After the oil layer was washed with 20 mL of a saturated aqueous sodium hydrogen carbonate solution, a separation was conducted. The oil layer was dried over 5 g of anhydrous sodium sulfate. This reaction liquid was filtered and the obtained filtrate was concentrated. The residue was purified by column chromatography (alumina neutral, hexane:ethyl acetate = 10:1 (v/v)) to yield 339 mg of a white powder of 1,1-bis[2-[(4S)-tert-butyloxazoline]]cyclopropane. Yield: 77%. 1H-NMR (δ: ppm, CD3Cl3 solvent, TMS standard) 4.22-4.10 (m, 4H), 3.85-3.79 (m, 2H), 1.52-1.48 (m, 2H), 1.29-1.24 (m, 2H), 0.86 (s, 18H); Example 7-3
References:
EP1698617,2006,A1 Location in patent:Page/Page column 12-13

132098-54-5
52 suppliers
$54.00/100mg

106-93-4
379 suppliers
$15.00/5g
![(4S,4'S)-2,2'-Cyclopropylidenebis[4-tert-butyl-4,5-dihydro
oxazole],99%e.e.](/CAS/20180527/GIF/195379-09-0.gif)
195379-09-0
25 suppliers
inquiry
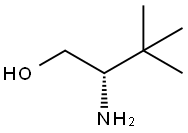
112245-13-3
267 suppliers
$10.00/250mg
![(4S,4'S)-2,2'-Cyclopropylidenebis[4-tert-butyl-4,5-dihydro
oxazole],99%e.e.](/CAS/20180527/GIF/195379-09-0.gif)
195379-09-0
25 suppliers
inquiry
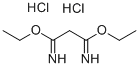
10344-69-1
67 suppliers
$14.00/250mg
![(4S,4'S)-2,2'-Cyclopropylidenebis[4-tert-butyl-4,5-dihydro
oxazole],99%e.e.](/CAS/20180527/GIF/195379-09-0.gif)
195379-09-0
25 suppliers
inquiry
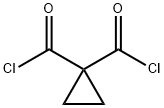
34782-60-0
6 suppliers
inquiry
![(4S,4'S)-2,2'-Cyclopropylidenebis[4-tert-butyl-4,5-dihydro
oxazole],99%e.e.](/CAS/20180527/GIF/195379-09-0.gif)
195379-09-0
25 suppliers
inquiry