
.beta.-D-Ribofuranoside, 1-naphthalenyl, tribenzoate synthesis
- Product Name:.beta.-D-Ribofuranoside, 1-naphthalenyl, tribenzoate
- CAS Number:195385-82-1
- Molecular formula:
- Molecular Weight:0
6974-32-9
437 suppliers
$6.00/5g
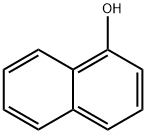
90-15-3
698 suppliers
$9.00/5g
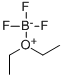
109-63-7
382 suppliers
$10.60/1gm:

195385-82-1
1 suppliers
inquiry
Yield:195385-82-1 23%
Reaction Conditions:
in dichloromethane;
Steps:
2.1 Stage 1.
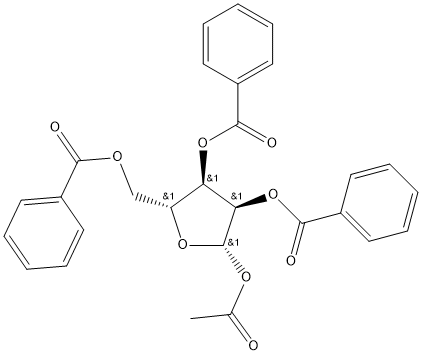
Stage 1. Preparation of α-naphthyl 2,3,5-tri-O-benzoyl-β-D-ribofuranoside A solution 1-O-acetyl-2,3,5-tri-O-benzoyl-β-D-ribofuranose (500 mg, 0.99 mmol), α-naphthol (428 mg, 3.00 mmol) and boron trifluoride diethyletherate (141 mg, 0.99 mmol. 125 μl) in dry dichloromethane (5 ml) was stirred at room temperature under argon for 16 hours. The mixture was worked up as for Stage 1 of Example 1 and purified by flash chromatography on silica gel (eluding with dichloromethane:hexanes, 3:2) to afford the title compound contaminated with α-naphthol. The latter was removed by dissolving the residue in ethyl acetate and washing with 10% aqueous sodium hydroxide solution, which after conventional processing, gave 136 mg, 23% of product as a yellow gum.
References:
US2001/19823,2001,A1