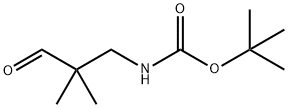
Carbamic acid, (2,2-dimethyl-3-oxopropyl)-, 1,1-dimethylethyl ester (9CI) synthesis
- Product Name:Carbamic acid, (2,2-dimethyl-3-oxopropyl)-, 1,1-dimethylethyl ester (9CI)
- CAS Number:195387-13-4
- Molecular formula:C10H19NO3
- Molecular Weight:201.26
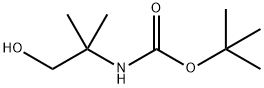
102520-97-8
116 suppliers
$7.00/1g

195387-13-4
18 suppliers
$310.00/100mg
Yield:195387-13-4 95.1%
Reaction Conditions:
with sodium hypochlorite;2,2,6,6-Tetramethyl-1-piperidinyloxy free radical;potassium bromide in dichloromethane;water at -10 - 0; for 0.583333 h;NaHCO3 buffer;
Steps:
51
Procedure 18: Synthesis of Aldehydes via TEMPO/Bleach OxidationTo a vigorously stirring solution of the alcohol (1.54 mmol) in DCM (4 mL) was added TEMPO (0.007 g, 0.045 mmol, 0.03 mol %) and a 2M aqueous KBr solution (75 mL, 0.15 mmol, 10 mol %) and the reaction mixture was cooled to -10°C.In a separate flask NaHCO3 (0.5 g, 9.5 mmol) was dissolved in bleach (25 mL, Chlorox 6.0% NaOCl) to yield a 0.78 M buffered NaOCl solution. This freshly prepared 0.78 MNaOCl solution (2.3 mL, 1.8 mmol, 117 mol %) was added to the reaction mixture over
References:
WO2009/67692,2009,A1 Location in patent:Page/Page column 45-46; 179
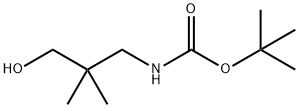
184357-44-6
46 suppliers
$60.00/100mg

195387-13-4
18 suppliers
$310.00/100mg
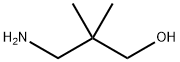
26734-09-8
114 suppliers
$35.28/1g

195387-13-4
18 suppliers
$310.00/100mg

24424-99-5
831 suppliers
$13.50/25G

195387-13-4
18 suppliers
$310.00/100mg