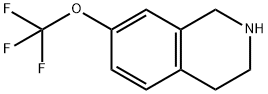
1,2,3,4-tetrahydro-7-(trifluoromethoxy)isoquinoline synthesis
- Product Name:1,2,3,4-tetrahydro-7-(trifluoromethoxy)isoquinoline
- CAS Number:199678-30-3
- Molecular formula:C10H10F3NO
- Molecular Weight:217.19
![Ethanone, 1-[3,4-dihydro-7-(trifluoromethoxy)-2(1H)-isoquinolinyl]-2,2,2-trifluoro-](/CAS/20210305/GIF/203506-18-7.gif)
203506-18-7
0 suppliers
inquiry

199678-30-3
27 suppliers
inquiry
Yield:199678-30-3 81.7%
Reaction Conditions:
with ethanol;water;potassium carbonate for 1.5 h;Heating / reflux;
Steps:
12.D
The amide (Step C, Intermediate 12) (2.37 g, 7.57 mmol) was dissolved in EtOH (25 mL) before a solution of K2C03 (5.23 g, 37.9 mmol) in H20 (25 mL) was added. The reaction mixture was refluxed for 1 1/2 hours before concentrated ill vacuo. The concentrate was diluted with H20 and extracted with DCM (5x). Combined organic layer was dried over MgS04, filtered, and concentrated to yield the title compound (1.34 g, 81.7%). %). 1H NMR (400MHz, CDC13) F 7.11 (d, J=8.4 Hz, 1H), 7.01 (bd, J=8.4 Hz, 1H), 6.89 (s, 1H), 4.03 (s, 2H), 3.15 (t, J=6.1 Hz, 2H), 2.80 (t, J=5.6 Hz, 2H), 1.80 (s, 1H).
References:
WO2003/93231,2003,A2 Location in patent:Page/Page column 88