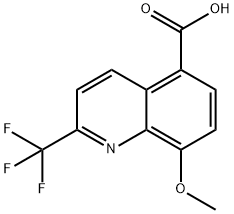
8-Methoxy-2-(trifluoroMethyl)quinoline-5-carboxylic acid synthesis
- Product Name:8-Methoxy-2-(trifluoroMethyl)quinoline-5-carboxylic acid
- CAS Number:199872-29-2
- Molecular formula:C12H8F3NO3
- Molecular Weight:271.19
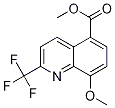
848685-30-3
1 suppliers
inquiry

199872-29-2
31 suppliers
$115.00/100mg
Yield:199872-29-2 98%
Reaction Conditions:
Stage #1: methyl 8-methoxy-2-trifluoromethyl-5-quinolinecarboxylatewith lithium hydroxide;water in tetrahydrofuran at 20; for 7 h;
Stage #2: with hydrogenchloride;water in ethyl acetate;
Steps:
3
Example 3. Preparation of the compound of formula IX :; To a mixture of the quinoline VIII (600 g, 2.1 mol) in 4 L THF at room temperature, a solution of LIOH monohydrate (180 g, 4.3 mol) in 3 L of H20 was added. After stirring at room temperature for 7 hours, the solvent THF was removed. EtOAc (12 L) was added followed by conc. HCI (330 mL). The layers were separated. The organic layer was dried over MGS04. REMOVAL of solvent gave the desired acid IX, 8-METHOXY-2-TRIFLUOROMETHYL-5- quinolinecarboxylic acid, (560 g, 98% yield). 1H NMR (400 MHz, DMSO) D 4.09 (s, 3H), 7.39 (d, J=8.4 Hz, 1H), 8. 11 (d, J=9.0 Hz, 1H), 8.45 (d, J=8.4 Hz, 1H), 9.71 (d, J=9.0 Hz, 1H), 13.25 (br, 1H)
References:
WO2005/28471,2005,A1 Location in patent:Page/Page column 22
41192-84-1
61 suppliers
$40.00/250mg

199872-29-2
31 suppliers
$115.00/100mg
41958-00-3
4 suppliers
inquiry

199872-29-2
31 suppliers
$115.00/100mg
201230-82-2
1 suppliers
inquiry
199872-02-1
1 suppliers
inquiry

199872-29-2
31 suppliers
$115.00/100mg