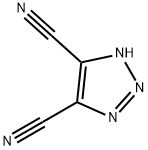
1H-1,2,3-triazole-4,5-dicarbonitrile synthesis
- Product Name:1H-1,2,3-triazole-4,5-dicarbonitrile
- CAS Number:53817-16-6
- Molecular formula:C4HN5
- Molecular Weight:119.08
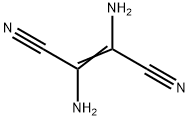
18514-52-8
26 suppliers
inquiry

53817-16-6
29 suppliers
inquiry
Yield:-
Reaction Conditions:
with sulfuric acid;sodium nitrite in water at 20 - 25; for 2 - 2.5 h;Product distribution / selectivity;
Steps:
1.2; 1
Step 2"; Synthesis of 4, 5-dicyanotriazolate silver; Into a flask equipped with a thermometer, a nitrogen gas inlet tube, a reflux condenser tube, a stirrer, and a dropping funnel, dicyanomaleonitrile (hereinafter, described as DAMN) 216 g (2.0 mol), sulfuric acid 98 g, and ion exchange water 400 g were charged. While the mixture was maintained at 0°C under nitrogen flow, sodium nitrile 138 g (2.0 mol) and water 400 g were added dropwise into the mixture over 1 hour.Then, the mixture was maintained at 25°C for 1 hour and then the reaction was completed.Then, the reaction solution was subjected to extraction step using diethyl ether and ion exchange water to obtain a brown
References:
WO2007/55392,2007,A1 Location in patent:Page/Page column 65-66; 72
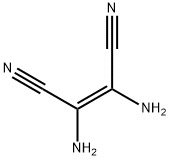
1187-42-4
336 suppliers
$6.00/5g

53817-16-6
29 suppliers
inquiry
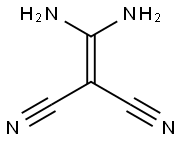
1187-12-8
5 suppliers
inquiry

53817-16-6
29 suppliers
inquiry