
1H-1,3-BENZIMIDAZOL-2-YLMETHYL PHENYL SULFIDE synthesis
- Product Name:1H-1,3-BENZIMIDAZOL-2-YLMETHYL PHENYL SULFIDE
- CAS Number:3176-72-5
- Molecular formula:C14H12N2S
- Molecular Weight:240.32
Yield: 92%
Reaction Conditions:
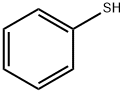
Stage #1:thiophenol with potassium carbonate for 2 h;Reflux;
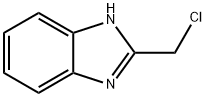
Stage #2:2-chloromethyl-1H-benzimidazole in N,N-dimethyl-formamide at 20; for 0.5 h;
Steps:
2-(phenylthiomethyl)-1H-benzo[d]imidazole (2)
A mixture solution of benzenethiol (20 mmol) and K2CO3 (20 mmol) in 10 ml of water was refluxed for 2 h, and evaporaed under vacuo, then dissolved 10 ml of DMF.2-(Chloromethyl)-1H-benzo[d]imidazole 1 (20 mmol), produced from o-phenylenediamine and 2-chloro- acetic acid by the procedures reported17, dissolved in dry DMF, was added to the mixture solution of potassium benzenethiolate as above. The mixture was stirred for 30 min at room temperature, which was then poured into ice water with stirring to result a solid product. The solid was collected by filteration, washed with water, and dried in vacuo to afford 2 as a white solid with 92% yield; mp 118-120 °C (Ref. [18]: mp 115 °C). 1H NMR (DMSO-d6): δ 4.44 (s, 2H, CH2), 7.13~7.19 (m, 3H, CH), 7.30 (m, 2H, CH), 7.41 (d, 2H, J = 7.6 Hz, CH), 7.48 (brs, 2H, CH).
References:
Liu, Ting;Sun, Changyan;Xing, Xiumei;Jing, Lingling;Tan, Rui;Luo, Youfu;Huang, Wencai;Song, Hang;Li, Zicheng;Zhao, Yinglan [Bioorganic and Medicinal Chemistry Letters,2012,vol. 22,# 9,p. 3122 - 3125] Location in patent:supporting information; experimental part
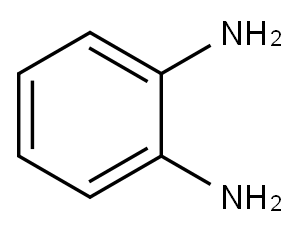
95-54-5
499 suppliers
$9.00/1g

3176-72-5
5 suppliers
inquiry

![2-(bromomethyl)-1H-benzo[d]imidazole](/CAS/20180703/GIF/30770-24-2.gif)