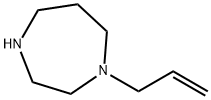
1H-1,4-Diazepine,hexahydro-1-(2-propenyl)-(9CI) synthesis
- Product Name:1H-1,4-Diazepine,hexahydro-1-(2-propenyl)-(9CI)
- CAS Number:229162-11-2
- Molecular formula:C8H16N2
- Molecular Weight:140.23

106-95-6
424 suppliers
$10.00/5g
112275-50-0
242 suppliers
$9.00/1g

229162-11-2
19 suppliers
$65.00/50mg
Yield:229162-11-2 58%
Reaction Conditions:
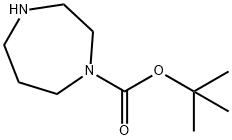
Stage #1: allyl bromide;tert-butyl 1,4-diazepine-1-carboxylate in dichloromethane at 25; for 2 h;
Stage #2: with trifluoroacetic acid in methanol;ethyl acetate at 20; for 1 h;
Steps:
175
To a solution of tert-butyl 1-homopiperazinecarboxylate (9.73 mL, 50 mmol) in dichloromethane (250 mL) was added PS-TBD resin (40 g, 100 mmol) and 3-bromoprop-1-ene (4.33 mL, 50 mmol) dropwise at 25° C. The mixture was stirred for 2 h. The PS-TBD was filtered off and the filtrate evaporated to dryness, redissolved in MeOH/EtOAc (1:9) and then filtered through a short silica column. The filtrate obtained was evaporated to dryness, treated with TFA (20 ml) and then stirred at room temperature for 1 h. The reaction mixture was evaporated to dryness and redissolved in MeOH (50 mL) and the crude product was purified by ion exchange chromatography, using an SCX column. The desired product was eluted from the column using 7M NH3/MeOH and evaporated to dryness to afford 1-prop-2-enyl-1,4-diazepane (4.07 g, 58.0%) as a pale yellow oil. 1H NMR (399.902 MHz, CDCl3) δ 1.70 (m, 2H), 1.98 (s, 1H), 2.59 (m, 4H), 2.85 (m, 4H), 3.06 (m, 2H), 5.07 (m, 2H), 5.81 (m, 1H)
References:
US2008/153812,2008,A1 Location in patent:Page/Page column 154