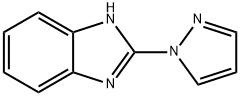
1H-Benzimidazole,2-(1H-pyrazol-1-yl)-(9CI) synthesis
- Product Name:1H-Benzimidazole,2-(1H-pyrazol-1-yl)-(9CI)
- CAS Number:6488-88-6
- Molecular formula:C10H8N4
- Molecular Weight:184.2
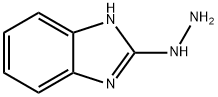
15108-18-6
87 suppliers
inquiry
102-52-3
347 suppliers
$5.00/10g

6488-88-6
15 suppliers
$202.00/500mg
Yield:6488-88-6 71%
Reaction Conditions:
with hydrogenchloride in water; for 2 h;Reflux;
Steps:
4.3.1 Synthesis of 2-(pyrazol-1-yl)-1H-benzimidazole L1
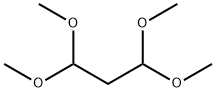
To a solution of 2-hydrazinobenzimidazole (750 mg;5mmol) in a mixture of 10mL of water and 0.75mL of concentrated HCl was gradually added 1,1,3,3-tetramethoxypropane(820mL; 5mmol). The resulting mixture was heated at reflux for 2 h, then was allowed to cool and filtered.The filtrate was neutralised with solid potassium carbonate,causing the product to precipitate as a pale tan solid, which was filtered and dried in vacuo. Yield 650mg (71%). m.p. 233-2358C; dH(500MHz, d6-DMSO) 6.66 (m, br, 1H,H2), 7.19-7.21 (m, 2H, H6 H7), 7.46 (d, 1H, J 6.1 Hz,H5), 7.58 (d, 1H, J 7.7 Hz, H8), 7.94 (d, 1H, J 1.5 Hz,H3), 8.59 (d, 1H, J 2.9 Hz, H1), 13.10 (s, br, 1H, H4);dC(125MHz, d6-DMSO) 109.2, 111.6, 118.4, 122.2, 122.5,129.1, 133.7, 141.7, 142.9, 146.1; m/z (HR-ES-MS)185.0821 ([M H], calculated for C10H9N4 185.0822);y max(KBr)/cm21 2943m br, 1628m, 1574s, 1479m, 1460s,1387m, 1324m, 1273m, 1226m, 1076s, 927s, 740s.
References:
Hawes, Chris S.;Kruger, Paul E. [Supramolecular Chemistry,2015,vol. 27,# 11-12,p. 757 - 771]
288-13-1
414 suppliers
$10.00/1g
32315-10-9
412 suppliers
$10.00/1g
95-54-5
500 suppliers
$9.00/1g

6488-88-6
15 suppliers
$202.00/500mg

288-13-1
414 suppliers
$10.00/1g
615-16-7
379 suppliers
$8.00/10g

6488-88-6
15 suppliers
$202.00/500mg

288-13-1
414 suppliers
$10.00/1g
134469-07-1
24 suppliers
inquiry

6488-88-6
15 suppliers
$202.00/500mg
4857-06-1
280 suppliers
$6.00/1g

6488-88-6
15 suppliers
$202.00/500mg