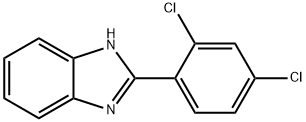
1H-BENZIMIDAZOLE, 2-(2,4-DICHLOROPHENYL)- synthesis
- Product Name:1H-BENZIMIDAZOLE, 2-(2,4-DICHLOROPHENYL)-
- CAS Number:14225-79-7
- Molecular formula:C13H8Cl2N2
- Molecular Weight:263.12
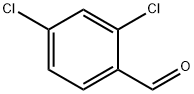
874-42-0
414 suppliers
$6.00/25g
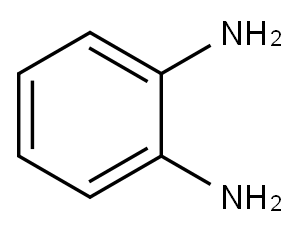
95-54-5
496 suppliers
$9.00/1g

14225-79-7
16 suppliers
$57.50/250mg
Yield:14225-79-7 98%
Reaction Conditions:
with manganese dioxide;silica gel in neat (no solvent) at 20; for 0.4 h;Milling;
Steps:
General Procedure for the Synthesis of Benzoimidazolesfrom o-phenylenediamine
General procedure: At the first, 0.017 g (20 mol %) of MnO2 nanoparticlesand 1g silica-gel were mixed in a mortar at room temperature.Then, o-phenylenediamine (1: 0.108 g; 1 mmol) andbenzaldehyde derivatives (2a: 0.11 ml, 2b: 0.12 ml, 2c: 0.12ml, 2d: 0.136 g, 2e: 0.166 g, 2f: 0.122 g, 2g: 0.138 g, 2h:0.172 g, 2i: 0.11 ml, 2j: 0.14 g, 2k: 0.175 g, 2l: 0.151 g, 2m:0.151 g, 2n: 0.185 g) were added to mixture in a mortar. Thereaction mixture was ground in the mortar for the appropriatetime as showed in Table 2. The progress of the reactionwas monitored by thin-layer chromatography (TLC). Aftercompletion of the reaction, ethyl acetate (4 ml) was added tomixture of the reaction, and then filtered off. Evaporation ofthe solvent gave the crude product. The pure product wasobtained by recrystallization from methanol/H2O (1:1). Theywere characterized by comparison of their physical and spectraldata with those of authentic samples [35-44].
References:
Naeimi, Hossein;Babaei, Zahra [Letters in Organic Chemistry,2015,vol. 12,# 5,p. 311 - 318] Location in patent:supporting information
1777-82-8
381 suppliers
$6.00/5g

95-54-5
496 suppliers
$9.00/1g

14225-79-7
16 suppliers
$57.50/250mg

56843-28-8
32 suppliers
$130.00/500mg

95-54-5
496 suppliers
$9.00/1g

14225-79-7
16 suppliers
$57.50/250mg
![N-[(2,4-dichlorophenyl)methylene]benzenamine](/CAS/20180629/GIF/5330-43-8.gif)