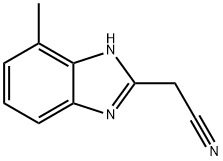
1H-Benzimidazole-2-acetonitrile,4-methyl-(9CI) synthesis
- Product Name:1H-Benzimidazole-2-acetonitrile,4-methyl-(9CI)
- CAS Number:55025-38-2
- Molecular formula:C10H9N3
- Molecular Weight:171.2

1379301-77-5
0 suppliers
inquiry

55025-38-2
18 suppliers
inquiry
Yield:55025-38-2 14%
Reaction Conditions:
with acetic acid for 4 h;Heating / reflux;
Steps:
177
With cooling with ice, 6.26 g (32.6 mmol) of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride was added to dichloromethane solution of 3.32 g (27.2 mmol) of 3-methylbenzene-1,2-diamine (I-176), 2.78 g (27.2 mmol) of cyanoacetic acid and 5.69ml (40.8 mmol) of triethylamine. After restoring to room temperature, 0.37 g (2.74 mmol) of 1-hydroxybenzotriazole was added thereto, and the resulting mixture was stirred at the temperature for 21 hours. Aqueous saturated sodium hydrogencarbonate solution was added to the reaction mixture, and the mixture was extracted with chloroform. The organic layer was washed with brine, and dried over anhydrous sodium sulfate. The solvent was evaporated under reduced pressure, and the residue was solidified with ethyl acetate/isopropyl ether to obtain 1.84 g of an amide compound as a crude brown crystal. This compound was dissolved in acetic acid (30 ml) and heated under reflux for 4 hours. After cooling, acetic acid was evaporated under reduced pressure. The residue wasdiluted with chloroform, washed with aqueous saturated sodium hydrogencarbonate solution and brine, and dried over anhydrous sodium sulfate. The solvent was evaporated under reduced pressure, and the residue was applied to a column chromatography. This was eluted with ethyl acetate to obtain a crude product of the entitled compound. The crystal was washed with isopropyl ether to obtain 666 mg (14 %) of the entitled compound as a brown crystal. MS(ESI)m/z:172(M+1)+.1H-NMR(CDCl3)δ: 2.47 and 2.52(total 3H, each s), 4.34 and 4.35(total 2H, each s), 7.00(1H, m), 7.08(1H, m), 7.28 and 7.41 (total 1H, each d, J=7.8 Hz) , 12.51 and 12.59(total 1H, each brs).
References:
EP1479681,2004,A1 Location in patent:Page 110; 111