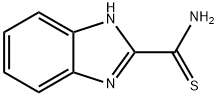
1H-Benzimidazole-2-carbothioamide(9CI) synthesis
- Product Name:1H-Benzimidazole-2-carbothioamide(9CI)
- CAS Number:35369-17-6
- Molecular formula:C8H7N3S
- Molecular Weight:177.23
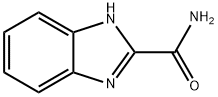
5805-52-7
56 suppliers
$82.00/50mg

35369-17-6
14 suppliers
inquiry
Yield:35369-17-6 61.2%
Reaction Conditions:
with Lawessons reagent in tetrahydrofuran at 66; for 5 h;
Steps:
1H-Benzo[d]imidazole-2-carbothioamide (10):
To a solution of 9 (3.0 g, 18.6 mmol) in THF (70 mL) wasadded Lawesson’s reagent (7.5 g, 18.6 mmol). The resulting solution was stirred at 66 °C for 5 h. Aftercomplete conversion of the starting material, the reaction mixture was concentrated in vacuo and theresidue was redissolved in DCM (500 mL), the solution was washed with saturated NaCl solutionthree times, dried over anhydrous sodium sulfate, concentrated and purified by flash silica gel columnchromatography (DCM:MeOH = 100:1) to obtain 10 as yellow solid in 61.2% yield. LC-MS m/z: 178.2[M + H]+.
References:
Wang, Shuxiang;Guan, Lihong;Zang, Jie;Xing, Kun;Zhang, Jian;Liu, Dan;Zhao, Linxiang [Molecules,2019,vol. 24,# 7,art. no. 1198]
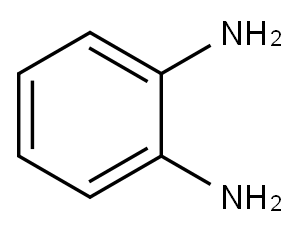
95-54-5
506 suppliers
$9.00/1g

35369-17-6
14 suppliers
inquiry
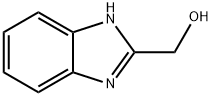
4856-97-7
234 suppliers
$6.00/1g

35369-17-6
14 suppliers
inquiry
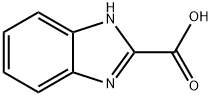
2849-93-6
191 suppliers
$6.00/250mg

35369-17-6
14 suppliers
inquiry
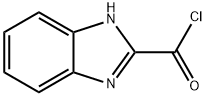
30183-14-3
7 suppliers
inquiry

35369-17-6
14 suppliers
inquiry