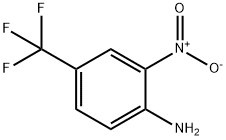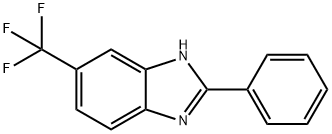
1H-BENZIMIDAZOLE, 2-PHENYL-5-(TRIFLUOROMETHYL)- synthesis
- Product Name:1H-BENZIMIDAZOLE, 2-PHENYL-5-(TRIFLUOROMETHYL)-
- CAS Number:91437-85-3
- Molecular formula:C14H9F3N2
- Molecular Weight:262.23
Yield:91437-85-3 74%
Reaction Conditions:
with 1,4-diaza-bicyclo[2.2.2]octane;octasulfur in neat (no solvent) at 140; for 24 h;Inert atmosphere;Sealed tube;
Steps:
1.2. Procedure for the synthesis of 6-methyl-2-phenylbenzimidazole
General procedure: In a typical experiment, a vial equipped with a magnetic stir bar was added with 4-methyl-2-nitroaniline (1aa, 76.1 mg, 0.5 mmol), benzyl alcohol (2aa, 270.4 mg, 2.5 mmol), elemental sulfur (48.0 mg, 1.5 mmol) and DABCO (56.1 mg, 0.5 mmol). The vial was purged with argon for 2 min, tightly sealed, heated to 140 oC at 10 oC/min, and held at this temperature for 24 h under vigorous stirring. Ethyl acetate (2 mL) was then added to dilute the reaction mixture. Aliquots withdrawn from the resulting mixture were quenched with water (1 mL), extracted with ethyl acetate (3 x 1 mL), dried over anhydrous sodium sulfate and added with diphenyl ether (0.5 mmol, 85 mg). The final organic sample was analyzed by GC to determine the product yield
References:
Le, Huy X.;Nguyen, Khoa D.;Phan, Nam T.S.;Le, Ha V.;Nguyen, Tung T. [Tetrahedron,2022,vol. 121,art. no. 132918] Location in patent:supporting information
![Benzenecarboximidamide, N-[2-nitro-4-(trifluoromethyl)phenyl]-](/CAS/20210305/GIF/1224442-16-3.gif)
1224442-16-3
0 suppliers
inquiry

91437-85-3
9 suppliers
inquiry