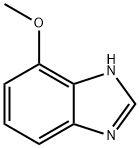
1H-BENZIMIDAZOLE, 4-METHOXY- synthesis
- Product Name:1H-BENZIMIDAZOLE, 4-METHOXY-
- CAS Number:27080-53-1
- Molecular formula:C8H8N2O
- Molecular Weight:148.16

64-18-6
1062 suppliers
$22.12/250ML
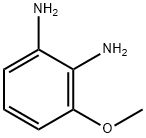
37466-89-0
88 suppliers
$30.00/100mg

27080-53-1
34 suppliers
$27.00/250mg
Yield:27080-53-1 83.9%
Reaction Conditions:
for 4 h;Reflux;
Steps:
6.1.4 4-Methoxy-1H-benzo[d]imidazole (3)
The reaction mixture of 3-methoxy-o-phenylenediamine (2, 190mg, 1.37mmol)and HCOOH (5mL) was refluxed for 4h, then cooled to room temperature and poured into ice/ water (20mL). The mixture was treated with 10% NaOH aqueous solution to pH≈8 and then extracted with ethyl ether (15mL×3). The combined extracts were washed with water and brine, then dried over anhydrous MgSO4. The organic phase was concentrated to give the crude product, which was purified by column chromatography with CH2Cl2-MeOH (v/v=60/1). The desired product 4-methoxy-1H-benzo[d]imidazole (162mg) was afforded as white powder with 83.9% yield. M.p. 165-167°C; 1H NMR (acetone-d6, 300MHz, δ ppm): 8.08 (1H, s), 7.21 (1H, d, J=7.8Hz), 7.11 (1H, t, J=7.8Hz), 6.73 (1H, d, J=7.8Hz), 3.97 (3H, s); HRMS (ESI): m/z, calcd. For C8H9N2O [M+H]+ 149.0709 found 149.0707.
References:
Zhou, Jie;Jin, Jing;Zhang, Yi;Yin, Yuwen;Chen, Xiaoguang;Xu, Bailing [European Journal of Medicinal Chemistry,2013,vol. 68,p. 222 - 232]

37466-89-0
88 suppliers
$30.00/100mg

64-19-7
1536 suppliers
$10.00/25ML

27080-53-1
34 suppliers
$27.00/250mg

603-85-0
260 suppliers
$15.00/5g

27080-53-1
34 suppliers
$27.00/250mg
16554-45-3
135 suppliers
$16.00/1g

27080-53-1
34 suppliers
$27.00/250mg

37466-89-0
88 suppliers
$30.00/100mg
141-53-7
716 suppliers
$6.00/100g

27080-53-1
34 suppliers
$27.00/250mg