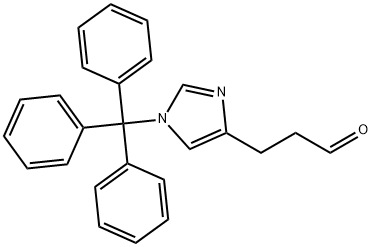
1H-Imidazole-4-propanal, 1-(triphenylmethyl)- synthesis
- Product Name:1H-Imidazole-4-propanal, 1-(triphenylmethyl)-
- CAS Number:102676-61-9
- Molecular formula:C25H22N2O
- Molecular Weight:366.45

79-37-8
463 suppliers
$17.67/10gm:

152030-49-4
13 suppliers
$486.00/1g

102676-61-9
10 suppliers
$189.28/250mg
Yield:102676-61-9 81%
Reaction Conditions:
with triethylamine in 4-(dicyanomethylene)-2-methyl-6-(p-dimethylaminostyryl)-4H-pyran;dimethyl sulfoxide;
Steps:
1.c N-(4-Chlorobenzyl)-4-(1H-imidazol-4-yl)-1-butanesulfonamide
Step c. 3-[1-(triphenylmethyl)imidazol-4-yl]propanal. A solution of oxalyl chloride (1.75 ml, 20.1 mmol) in DCM (60 ml) was cooled to -78° C. and dimethylsulfoxide (2.85 ml, 20.1 mmol) was added dropwise, with concomitant effervescence. The solution was stirred for 5 min, by which time effervescence had ceased, and a solution of 3-[1-(triphenylmethyl)imidazol-4-yl]propan-1-ol (6.17 g, 16.7 mmol)1 in DCM (30 ml) was added by means of a cannula. The solution was stirred for 20 min, triethylamine (8.40 ml, 60.2 mmol) was added, the cold bath was removed and the resultant solution stirred for 3 h. A column of silica was flushed with an equal volume of ethyl acetate. The reaction mixture was applied to the top of the column and the column eluted to dryness. This was repeated with a column's volume of DCM to ensure complete removal of dimethylsulfide. The column was eluted with ethyl acetate and the aldehyde collected in fractions. The combined fractions were evaporated to give the product as a white solid (4.94 g, 81%).
References:
US6355665,2002,B1

152030-49-4
13 suppliers
$486.00/1g

102676-61-9
10 suppliers
$189.28/250mg
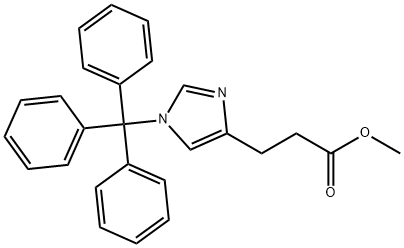
102676-60-8
25 suppliers
$130.00/250mg

102676-61-9
10 suppliers
$189.28/250mg

76-83-5
686 suppliers
$6.00/25g

102676-61-9
10 suppliers
$189.28/250mg

54260-89-8
5 suppliers
inquiry

102676-61-9
10 suppliers
$189.28/250mg