
1H-Indazole-3-carboxylicacid,4-chloro-,Methylester synthesis
- Product Name:1H-Indazole-3-carboxylicacid,4-chloro-,Methylester
- CAS Number:932041-14-0
- Molecular formula:C9H7ClN2O2
- Molecular Weight:210.62

67-56-1
746 suppliers
$9.00/25ml
201230-82-2
1 suppliers
inquiry
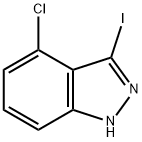
518990-33-5
51 suppliers
inquiry

932041-14-0
23 suppliers
inquiry
Yield:932041-14-0 70%
Reaction Conditions:
with bis-triphenylphosphine-palladium(II) chloride;triethylamine;4,5-bis(diphenylphosphino)-9,9-dimethylxanthene in dichloromethane at 50;
Steps:
JJ.3 Step 1: Synthesis of methyl 1H-pyrazolo [3 ,4-b]pyridine-3-carboxylate
General procedure: Similar to as described in General Procedure 0, 3-iodo-1H-pyrazolo[3,4-b]pyridine was reacted with carbon monoxide to give the title compound (418 mg, 62%) as a white solid. LC-MS (ES,m/z): 178 [M+H]’; To a nitrogen-purged solution of aryl iodide in TEA (3mL/mmol), DMF (3mL/mmol) and MeOH (3mL/mmol) was added Palladium (II)Acetate (0.03eq) and Xantphos (0.O6eq). The reaction mixture was flushed with Carbon Monoxide gas for several minutes and then sealed with CO balloon attached and heated to 60°C for 3 hours. Upon completion, the reaction was cooled to room temperature and the crude product was triterated via addition of water and collected byfiltration. The crude interemediate was taken into the next step w/o further purification.
References:
WO2015/25025,2015,A1 Location in patent:Page/Page column 103; 167
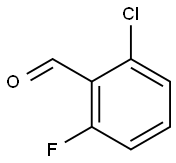
387-45-1
366 suppliers
$9.00/1g

932041-14-0
23 suppliers
inquiry
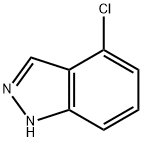
13096-96-3
146 suppliers
$15.00/250mg

932041-14-0
23 suppliers
inquiry