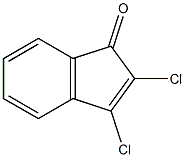
1H-Inden-1-one, 2,3-dichloro- synthesis
- Product Name:1H-Inden-1-one, 2,3-dichloro-
- CAS Number:876-82-4
- Molecular formula:C9H4Cl2O
- Molecular Weight:199.0335

1807-49-4
0 suppliers
inquiry

876-82-4
0 suppliers
inquiry
Yield:876-82-4 87%
Reaction Conditions:
with oxalyl dichloride in fluorobenzene at 50; for 3 h;
Steps:
2.5.2. General procedure for the large scale synthesis of α,β-dichloroenones
General procedure: AuFeAg-NPs (35 mg, 10.0 mol.%) were added to a solution ofdiazodicarbonyl compound 1a (10.0 mmol, 1.660 g) and oxalylchloride (15 mL) in PhF (10.0 mL) at room temperature. The reac-tion mixture was stirred at 50C for 3 h until the reaction wascompleted, as indicated by TLC. The solvent was evaporated underreduced pressure to give the residue. The residue was purified byflash column chromatography on silica gel using n-hexane/EtOAc(20:1) to give the product 2a (1.785 g, 93%).
References:
Mishra, Kanchan;Basavegowda, Nagaraj;Lee, Yong Rok [Applied Catalysis A: General,2015,vol. 506,p. 180 - 187]
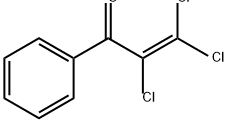
34907-91-0
0 suppliers
inquiry

876-82-4
0 suppliers
inquiry
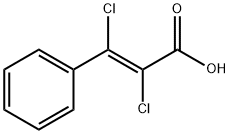
708-86-1
0 suppliers
inquiry

876-82-4
0 suppliers
inquiry
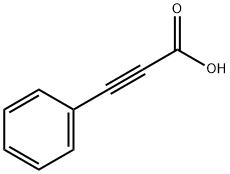
637-44-5
219 suppliers
$6.00/1g

876-82-4
0 suppliers
inquiry
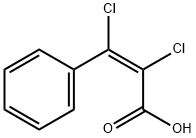
708-85-0
1 suppliers
inquiry

876-82-4
0 suppliers
inquiry