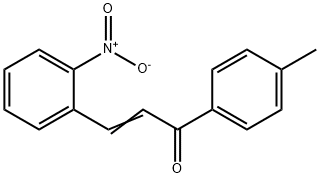
1H-Indol-2-yl(p-tolyl) ketone synthesis
- Product Name:1H-Indol-2-yl(p-tolyl) ketone
- CAS Number:1026-21-7
- Molecular formula:C16H13NO
- Molecular Weight:235.28
Yield:1026-21-7 95.4%
Reaction Conditions:
with 1-(diphenylphosphino)ferrocene-1-(di-tert-butylphosphino)ferrocene;1-cyanopropyl-3-methylimidazolium tetrafluoroborate;1,3,4,6,7,8-hexahydro-2H-pyrimido[1,2-a]pyrimidine;tin(IV) iodide;cerium triflate in ethylene glycol at 100; for 8 h;Reagent/catalyst;
Steps:
2 Example 2
To the appropriate amount of organic solvent (mass ratio of 1: 4 1-cyanopropyl-3-methylimidazolium tetrafluoroborate mixed with ethylene glycolCompound)Add lOOmmo 1 on the formula (I) compound,200 mmo 1 The compound of formula (II)5mmo 1 catalyst (for lmmol 1 -Diphenylphosphino-1 '- (di-tert-butylphosphino) ferrocene 4 mmol tin iodide mixture),250 mmol of alkali 1,5,7_ tri-nitrogenHeterobicyclo [4.4.0] dec-5-ene (TBD)And 10 mmol of cerium triflate (Ce (OTf) 3), and then heated to 100 ° CAnd the reaction was stirred at this temperature for 8 hours;After completion of the reaction, deionized water was added to the reaction system and thoroughly shaken, washed, and then extracted with chloroform 2-3 times,The combined organic phases were washed again with saturated brine, dried over anhydrous magnesium sulfate and concentrated under reduced pressure. The residue was passed over 300-400 meshSilica gel column chromatography eluting with a mixture of acetone and petroleum ether in a volume ratio of 1: 2 to give a compound of the above formula (III)The yield of the compound was 95.4%.
References:
CN105949109,2016,A Location in patent:Paragraph 0038-0041; 0054-0084
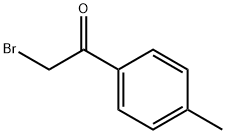
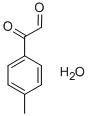
62322-75-2
0 suppliers
inquiry

1026-21-7
1 suppliers
inquiry
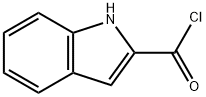
58881-45-1
24 suppliers
$141.38/500mgs:
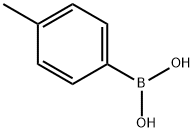
5720-05-8
443 suppliers
$5.00/1g

1026-21-7
1 suppliers
inquiry
![Benzenamine, 2-[(1E)-2-(4-methylphenyl)ethenyl]- (9CI)](/CAS/GIF/823809-34-3.gif)