
1H-Indole, 2-iodo-1-(phenylsulfonyl)- synthesis
- Product Name:1H-Indole, 2-iodo-1-(phenylsulfonyl)-
- CAS Number:99275-44-2
- Molecular formula:C14H10INO2S
- Molecular Weight:383.2
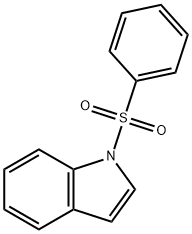
40899-71-6
151 suppliers
$5.00/1g

99275-44-2
7 suppliers
inquiry
Yield:99275-44-2 93%
Reaction Conditions:
with Iodine monochloride in dichloromethane at 20; for 1 h;Inert atmosphere;
Steps:
General method
General procedure: A solution of indole, azaindole or pyrrole (0.1 mmol) in dichloromethane (5 mL) was added to a suspension of 1:1 (W/W) ICl (concentration indicated in the Table; 0.1 mmol = 162 mg) and Celite (162 mg) ratio in dichloromethane (10 mL). The mixture was stirred at room temperature for the time indicated in Tables 1-4. The reaction mixture was then poured into saturated thiosulfate solution (20 mL) extracted with dichloromethane (2 x 15 mL). The organic layer was washed in brine and, dried over sodium sulfate and the solvent was removed at reduced pressure. The desired compound was purified by chromatography column using hexane and ethyl acetate mixtures as eluting solvent. Yields of iodinated compounds are summarized in Tables 1-4. All the compounds were characterized by IR, NMR and were compared with authentic samples.
References:
Hamri, Salha;Rodríguez, Jose;Basset, Joan;Guillaumet, Gérald;Dolors Pujol [Tetrahedron,2012,vol. 68,# 31,p. 6269 - 6275] Location in patent:supporting information; experimental part

40899-71-6
151 suppliers
$5.00/1g

99275-44-2
7 suppliers
inquiry
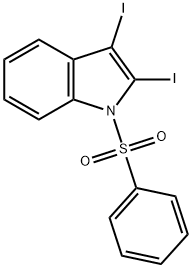
80360-26-5
12 suppliers
$504.06/5MG
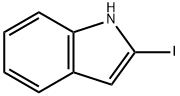
26340-49-8
60 suppliers
$109.00/100mg
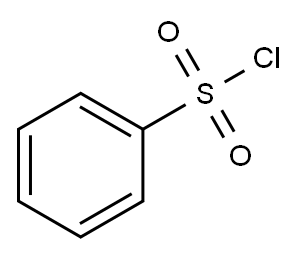
98-09-9
356 suppliers
$12.00/25g

99275-44-2
7 suppliers
inquiry
120-72-9
646 suppliers
$6.00/25g

99275-44-2
7 suppliers
inquiry

98-09-9
356 suppliers
$12.00/25g

99275-44-2
7 suppliers
inquiry