
1H-Indole-2-methanol, 6-fluoro- synthesis
- Product Name:1H-Indole-2-methanol, 6-fluoro-
- CAS Number:884048-32-2
- Molecular formula:C9H8FNO
- Molecular Weight:165.16
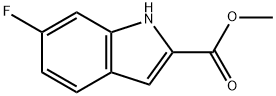
136818-43-4
60 suppliers
$132.00/0.5g

884048-32-2
6 suppliers
inquiry
Yield:884048-32-2 86.2%
Reaction Conditions:
with lithium aluminium tetrahydride in tetrahydrofuran at 0; for 2 h;
Steps:
Experimental procedure for the synthesis of (6-fluoro-1H-indol-2-yl)-methanol
In a 100 ml_ round-bottom flask methyl 6-fluoro-1 H-indole-2-carboxylate (250 mg; 1.229 mmol) is taken up in dry THF (20 ml_) and cooled to 0°C in an ice-bath. Then LAH (737.68 pi; 1.475 mmol) is added dropwise and the mixture is stirred at 0°C for 2 h. The reaction mixture is cautiously quenched with water, acidified with some drops of 4N HCI and extracted with DCM twice. The combined organic layers are concentrated under reduced pressure. The residue is dissolved in ACN/H2O, filtered through a syringe filter and purified via prep. RP-HPLC(Gilson; column: SunFire Prep C18, 10pm (50*150), gradient: ACN/water, basic conditions, 10-70% ACN in 9 min. flowrate: 150 mL/min wavelength: 218 nm). The product containing fractions are pooled and freeze dried to yield (6-fluoro-1 H- indol-2-yl)-methanol (175 mg; 1.06 mmol; 86.2 %). HPLC method: LCMSBAS1 : [M+H]+ = 166; tret [min] = 0.87 min
References:
WO2020/173935,2020,A1 Location in patent:Page/Page column 66

348-37-8
129 suppliers
inquiry

884048-32-2
6 suppliers
inquiry
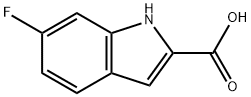
3093-97-8
167 suppliers
$13.00/250mg

884048-32-2
6 suppliers
inquiry

399-51-9
343 suppliers
$11.00/5g

884048-32-2
6 suppliers
inquiry