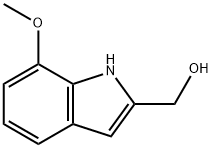
1H-Indole-2-methanol, 7-methoxy- synthesis
- Product Name:1H-Indole-2-methanol, 7-methoxy-
- CAS Number:30464-83-6
- Molecular formula:C10H11NO2
- Molecular Weight:177.2
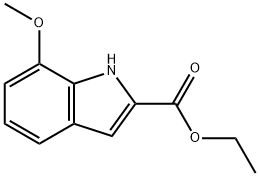
20538-12-9
32 suppliers
$51.00/100mg

30464-83-6
10 suppliers
inquiry
Yield:30464-83-6 10 g
Reaction Conditions:
with lithium aluminium tetrahydride in tetrahydrofuran at 0 - 25; for 3 h;
Steps:
35A Embodiment 35A
(7-Methoxy-1H-indol-2-yl)methanol
Ethyl-7-methoxy-1H-indole-2-carboxylate (11.00 g, 50.17 mmol) was dissolved in THF (100 mL) at 0 °C and lithium aluminum hydride (2.86 g, 75.26 mmol) was added to the mixture and the reaction mixture was warmed to 25 °C and stirred for 3 hours. LCMS showed the reaction was complete, H2O (1 mL) and NaOH (1 mL) were added to the reaction mixture, then H2O (3 mL) was added, filtered and the filtrate was concentrated to deliver the title compound (brown oil, 10.00 g).
1H NMR (400MHz, CDCl3): δ 8.57 (br. s., 1 H), 7.20 (d, J=8.0 Hz, 1 H), 7.03 (t, J=8.0 Hz, 1 H), 6.65 (d, J=8.0 Hz, 1 H), 6.40 (d, J=4.0 Hz, 1 H), 4.84 (d, J=4.0 Hz, 2 H), 3.97 (s, 3 H). LCMS (ESI) (5-95AB): m/z: 178.1 [M+1].
References:
EP3290419,2018,A1 Location in patent:Paragraph 0494; 0495; 0496
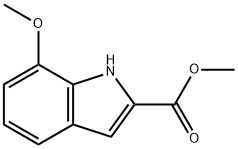
84638-71-1
22 suppliers
inquiry

30464-83-6
10 suppliers
inquiry