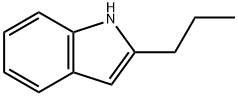
1H-Indole, 2-propyl- synthesis
- Product Name:1H-Indole, 2-propyl-
- CAS Number:13228-41-6
- Molecular formula:C11H13N
- Molecular Weight:159.23
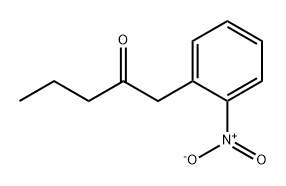
85355-51-7
0 suppliers
inquiry

13228-41-6
5 suppliers
inquiry
Yield:13228-41-6 42%
Reaction Conditions:
with palladium 10% on activated carbon;hydrogen in ethanol at 20;
Steps:
13B.4
1-(2-Nitrophenyl)pentan-2-one (0.31 g, 1.5 mmol) obtained in step 3 was dissolved in ethanol (3.0 mL), 10% palladium carbon (0.032 mg, 0.30 mmol) was added, and the mixture was stirred at room temperature overnight under a hydrogen atmosphere. The reaction solution was filtered through celite, and the filtrate was concentrated under reduced pressure. The obtained reaction residue was purified by silica gel column chromatography (hexane/ethyl acetate = 10/1 v/v) to give 2-propyl-1H-indole (100 mg, 42%). ESIMS m/z: 160 (M + H)+; 1H NMR (270 MHz, CDCl3, δ): 1.03 (t, J = 7.3 Hz, 3H), 1.71-1.80 (m, 2H), 2.74 (t, J = 7.3 Hz, 2H), 6.21 (br s, 1H), 7.04-7.12 (m, 2H), 7.30 (d, J = 7.8 Hz, 1H), 7.52 (d, J= 7.8 Hz, 1H), 7.86 (br s, 1H)
References:
EP2740730,2014,A1 Location in patent:Paragraph 0386

91133-05-0
0 suppliers
inquiry

13228-41-6
5 suppliers
inquiry
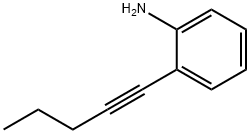
26059-38-1
0 suppliers
inquiry

13228-41-6
5 suppliers
inquiry
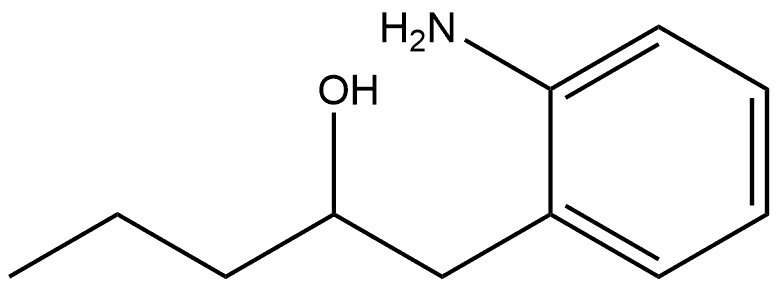
1035225-98-9
0 suppliers
inquiry

13228-41-6
5 suppliers
inquiry
![Acetamide, N-[2-(1-pentyn-1-yl)phenyl]-](/CAS/20210305/GIF/90585-00-5.gif)
90585-00-5
0 suppliers
inquiry

13228-41-6
5 suppliers
inquiry