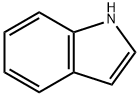
1H-Indole-3-ethanol, β-1H-indol-3-yl- synthesis
- Product Name:1H-Indole-3-ethanol, β-1H-indol-3-yl-
- CAS Number:95331-90-1
- Molecular formula:C18H16N2O
- Molecular Weight:276.34
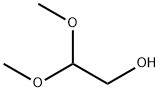
3616-48-6
3 suppliers
inquiry

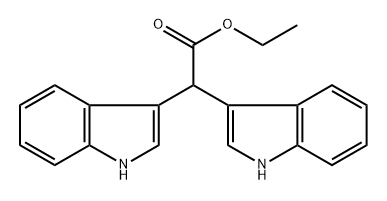
95331-90-1
3 suppliers
inquiry
Yield:95331-90-1 74%
Reaction Conditions:
with lithium aluminium tetrahydride in tetrahydrofuran at 0 - 20; for 3 h;
Steps:
3.1 Example 3: Compounds 2,2-bis(1H-indol-3-yl)ethanol (I-5) and 2,2-bis(1H-indol-3-yl)ethyl acetate (I- 6) Synthesis:
The first step: 0, while stirring, slowly add lithium aluminum tetrahydrogen (0.57g, 15mmol) to tetrahydrofuran (50mL), stir for 10min, slowly add ester I-1 (1.59g, 5mmol), the reaction solution is natural The temperature was raised to room temperature and reacted for 3 hours. Water (10 mL) was slowly added dropwise to the reaction system to quench the reaction. Add diatomaceous earth to suction filtration, wash the filter cake with ethyl acetate, combine the organic phases and dry with anhydrous Na2SO4, suction filtration, concentrate, and column chromatography to obtain light yellow oily compound I-5 (1.02g, 74%);
References:
CN113040151,2021,A Location in patent:Paragraph 0027-0028
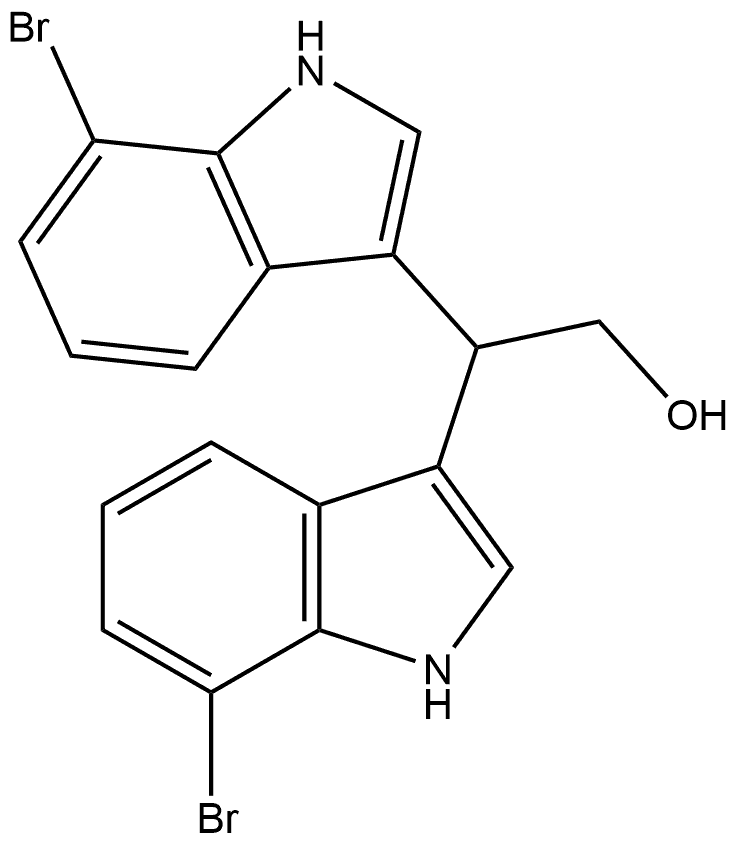
1445721-33-4
0 suppliers
inquiry

95331-90-1
3 suppliers
inquiry
![1H-Indole, 3,3'-[2-(phenylmethoxy)ethylidene]bis-](/CAS/20210305/GIF/200347-50-8.gif)
200347-50-8
0 suppliers
inquiry

95331-90-1
3 suppliers
inquiry

78205-43-3
0 suppliers
inquiry

95331-90-1
3 suppliers
inquiry