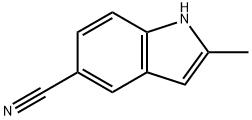
1H-Indole-5-carbonitrile,2-methyl- synthesis
- Product Name:1H-Indole-5-carbonitrile,2-methyl-
- CAS Number:36798-24-0
- Molecular formula:C10H8N2
- Molecular Weight:156.18

1240112-40-6
0 suppliers
inquiry

36798-24-0
29 suppliers
inquiry
Yield:36798-24-0 93%
Reaction Conditions:
with methanol;sodium hydroxide in tetrahydrofuran at 0 - 20;
Steps:
71
Preparation 71: 2-Methyl-lH-indole-5-carbonitrile; [824] l-(Benzenesulfonyl)-2-methyl-indole-5-carbonitrile (41.33g, 139mmol) obtained in Preparation 70 was dissolved in 25OmL of tetrahydrofuran and 25OmL of methanol, and the mixture was cooled to O0C. 14OmL of ION sodium hydroxide was slowly added in drops, and the mixture was stirred for 30 min at O0C. The mixture was slowly warmed to room temperature over 15 h while stirring. After completion of the reaction, water was added. The solid compound was filtered, washed with water, and dried to give 20.39g (131mmol, Yield 93%) of the title compound.[825] NMR 1H-NMR(CDCl3) δ 8.23(1H, br), 7.84(1H, s), 7,34(2H, m), 6.29(1H, s), 2.48(3H, s)[826] Mass(EI): 157(M++1)
References:
WO2010/93191,2010,A2 Location in patent:Paragraph 822-826
1075-34-9
149 suppliers
$8.00/100mg

124-38-9
130 suppliers
$214.00/14L

36798-24-0
29 suppliers
inquiry

873-74-5
650 suppliers
$9.00/1g

36798-24-0
29 suppliers
inquiry