
1H-Indole-6-carboxylic acid, 7-chloro- synthesis
- Product Name:1H-Indole-6-carboxylic acid, 7-chloro-
- CAS Number:1055320-72-3
- Molecular formula:C9H6ClNO2
- Molecular Weight:195.6
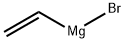
1826-67-1
213 suppliers
$60.89/100ml
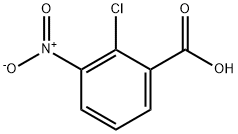
3970-35-2
285 suppliers
$6.00/250mg

1055320-72-3
27 suppliers
$1189.00/500mg
Yield:1055320-72-3 90%
Reaction Conditions:
Stage #1: vinyl magnesium bromide;2-chloro-3-nitrobezoic acid in tetrahydrofuran at -45 - 20;Cooling with ethanol-dry ice;
Stage #2: with water;ammonium chloride in tetrahydrofuran at 0; for 1 h;
Stage #3: with hydrogenchloride in water; pH=2;
Steps:
7-chloro-1H-indole-6-carboxylic acid. A two liter 3-neck flask was assembled with an overhead stir, flame dried and flushed with nitrogen. To this was added 1.0 equivalent of 2-chloro-3-nitrobenzoic acid (30 grams, 148.8 mmol) in 300 mL dry THF. The flask was then cooled to -45° C. with the aid of an ethanol/dry ice bath. 4.0 equivalents of cold Vinyl Grignard reagent (Aldrich, 1M Sure/Seal bottles) was then cannulated into the solution of nitrobenzoic acid. An additional 100 mL of dry THF was then added via cannula to the very thick solution. The flask was allowed to slowly warm to room temperature overnight. In the morning, the flask was cooled to 0° C. with an ice bath and 400 mL of saturated NH4Cl was added in four portions. The mixture was stirred for one hour then transferred to a separatory funnel and extracted with ethyl acetate (300 mL×3). The organic layers were combined, washed with brine (100 mL), dried over MgSO4 and evaporated to dryness on the rotovap. The aqueous layer was acidified to pH=2 with 2M HCl. The resulting solid was filtered, combined with the above isolated solid and dried in a vacuum desiccator over night to yield 26.32 grams (90%) of a tan powder that was used without further purification. 1H NMR (300 MHz): (CD3OD) δ 7.59 (d, J=8.42 Hz, 1H), 7.49 (d, J=8.42 Hz, 1H), 7.42 (d, J=2.93 Hz, 1H), 6.53 (d, J=2.93 Hz, 1H); Analytical HPLC method: Solvent A=10% MeOH-90% H2O-0.1% TFA, Solvent B=90% MeOH-10% H2O-0.1% TFA, Start % B=0, Final % B=100, Gradient time=2 min, Flow Rate=5 ml/min, Column: Xterra MS C18 S7 3.0×50 mm; LC/MS: (ES+) m/z (M+H)+=195.92, 197.92, HPLC Rt=1.018 min.
References:
US2008/221090,2008,A1 Location in patent:Page/Page column 16-17