
1H-Indole, 7-broMo-5-Methyl- synthesis
- Product Name:1H-Indole, 7-broMo-5-Methyl-
- CAS Number:15936-79-5
- Molecular formula:C9H8BrN
- Molecular Weight:210.07
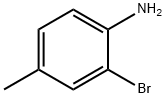
583-68-6
292 suppliers
$10.00/5G

107-14-2
290 suppliers
$15.00/25g

107-06-2
438 suppliers
$14.00/25g

15936-79-5
33 suppliers
$164.00/1g
Yield:15936-79-5 90%
Reaction Conditions:
with hydrogenchloride;sodium borohydrid;boron trichloride;aluminium trichloride in 1,4-dioxane;dichloromethane;water;
Steps:
25.a 1,5-Dimethyl-5H,13H-indolo[6,7-a]pyrrolo[3,4-c]carbazole-6,8-dione
(a) 5-Methyl-7-bromoindole. 2-Bromo-4-methyl-aniline (10.0 g, 53.7 mmol) was added dropwise to a solution of boron trichloride in methylene chloride (1.0 M, 60 mL, 60 mmol) cooled with ice water. The reaction mixture was warmed to room temperature, stirred for 30 min, and chloroacetonitrile (10 mL, 64.4 mmol) and aluminum chloride (10 g, 59.8 mmol) added, followed by 1,2-dichloroethane (70 mL). The reaction mixture was heated to 70° C. to distill off methylene chloride, and then refluxed for 24 hrs. The mixture was cooled to 0-5° C., treated with 25 M HCl (96 mL) at 0~5° C. carefully, and then heated to 80° C. for 1 h until all solids dissolved. The aqueous layer was separated and extracted with methylene chloride. The combined organic layers were washed with water and brine, dried (sodium sulfate) and concentrated a yellow solid (11.6 g, 82.3%), that was used without-further purification. The crude product was taken into dioxane (76 mL) and water (8.5 mL), and treated with sodium borohydride (1.75 g, 46.3 mmol) in portions. After 30 min at room temperature, all the starting material was consumed, and the reaction mixture was heated to reflux for 14 h. The mixture was then cooled to room temperature, treated with 0.1 N HCl (74 mL), and extracted with ethyl acetate. The extract was washed with water and brine, dried (Na2SO4) and concentrated. Column chromatography on silica gel (hexanes/ethyl acetate) gave 5-methyl-7-bromoindole (8.0 g, 90%). 1H NMR (400 MHz, CDCl3) 2.43 (s, 3H), 6.54 (dd, J1=1.94 Hz, J2=3.13 Hz, 1H), 7.20 (d, J=0.78 Hz, 1H), 7.22 (m, 1H), 7.36 (m, 1H), 8.22 (br, 1H); MS (ES, m/z): C9H8BrN: 210 (M+(79Br)+1), 212.0 (M+(81Br)+1).
References:
US2004/48915,2004,A1

40385-54-4
78 suppliers
$15.00/250mg
1826-67-1
215 suppliers
$60.89/100ml

15936-79-5
33 suppliers
$164.00/1g

583-68-6
292 suppliers
$10.00/5G

15936-79-5
33 suppliers
$164.00/1g

289038-12-6
17 suppliers
inquiry

15936-79-5
33 suppliers
$164.00/1g