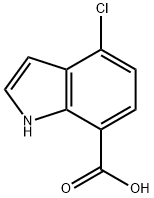
1H-Indole-7-carboxylic acid, 4-chloro- synthesis
- Product Name:1H-Indole-7-carboxylic acid, 4-chloro-
- CAS Number:875305-77-4
- Molecular formula:C9H6ClNO2
- Molecular Weight:195.6

124-38-9
122 suppliers
$214.00/14L
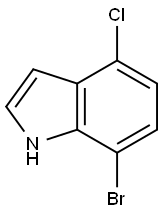
126811-29-8
76 suppliers
$69.00/100mg

875305-77-4
38 suppliers
$108.00/100mg
Yield:875305-77-4 67%
Reaction Conditions:
Stage #1: 7-bromo-4-chloro-1H-indolewith n-butyllithium in tetrahydrofuran at -78 - 5; for 0.5 h;
Stage #2: carbon dioxide in tetrahydrofuran at -78 - 20; for 0.25 h;
Steps:
S65
560 mg (2.43 mmol) of 7-bromo-4-chloro-1H-indole were dissolved in 15 ml THF. The reaction mixture was cooled to -78° C. and 4.55 ml of a 1.6 M butyl lithium solution in hexane were added under nitrogen in such a way, that the temperature did not exceed a maximum of -70° C. The yellow solution was stirred at 0 to 5° C. after complete addition for 30 min. The reaction mixture was cooled to -78° C. and dry ice was added. The reaction mixture was warmed to rt, stirred for 15 min and poured onto 100 ml water. The aqueous layer was washed twice with diethyl ether, acidified with 1N aqueous HCl solution and extracted twice with diethyl ether. The combined organic layers were washed with saturated aqueous sodium chloride solution, dried over sodium sulfate, filtered and the solvent was evaporated. The crude residue was stirred with hexane for 15 min, filtered and dried to yield 319 mg (67%) of 4-chloro-1H-indole-7-carboxylic acid as an off-white solid. MS (ISP) 194.1 (M-H)-.
References:
US2006/30613,2006,A1 Location in patent:Page/Page column 35

41513-04-6
252 suppliers
$6.00/5g

875305-77-4
38 suppliers
$108.00/100mg

126811-29-8
76 suppliers
$69.00/100mg

875305-77-4
38 suppliers
$108.00/100mg