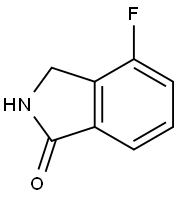
1H-Isoindol-1-one,4-fluoro-2,3-dihydro-(9CI) synthesis
- Product Name:1H-Isoindol-1-one,4-fluoro-2,3-dihydro-(9CI)
- CAS Number:366452-96-2
- Molecular formula:C8H6FNO
- Molecular Weight:151.14

1192042-15-1
0 suppliers
inquiry

366452-96-2
34 suppliers
$45.00/10mg
Yield:-
Reaction Conditions:
with triethylsilane;trifluoroacetic acid in dichloromethane at 20;
Steps:
295A
7458 mg (12.1 mmol) of sodium borohydride were added a little at a time to a suspension, cooled to 0° C., of 4.0 g (24.2 mmol) of 4-fluoro-1H-isoindole-1,3(2H)-dione in 40 ml of ethanol. After the addition had ended, cooling was removed and the mixture was warmed to RT. After 20 min of stirring, a further 458 mg (12.1 mmol) of sodium borohydride were added at RT. After a further 30 min at RT, 1 N hydrochloric acid was added carefully to the reaction mixture and the pH was adjusted to neutral. The mixture was concentrated under reduced pressure and the residue was taken up in dichloromethane and filtered through Celite. The collected filtrate was concentrated under reduced pressure. This gave 4.05 g of 4-fluoro-3-hydroxy-2,3-dihydro-1H-isoindol-1-one, which was directly reacted further as crude product.4.05 g (about 24.2 mmol) of 4-fluoro-3-hydroxy-2,3-dihydro-1H-isoindol-1-one were initially charged in 10 ml of dichloromethane, 22.4 ml (290.8 mmol) of trifluoroacetic acid and 7.7 ml (48.5 mmol) of triethylsilane were added and the mixture was stirred at RT overnight. The reaction mixture was then concentrated and the residue was dissolved in dichloromethane and washed with sodium bicarbonate solution. The crude product was purified by chromatography on silica gel (mobile phase cyclohexane/ethyl acetate 3:1 to 1:1). This gave 890 mg (24.2% of theory) of the target compound.LC-MS (method 12): Rt=1.18 min; m/z=152 (M+H)+.1H-NMR (400 MHz, DMSO-d6): δ=8.77 (br. s, 1H), 7.58-7.51 (m, 2H), 7.49-7.40 (m, 1H), 4.46 (s, 2H).
References:
US2011/34450,2011,A1 Location in patent:Page/Page column 114
201230-82-2
1 suppliers
inquiry
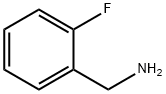
89-99-6
246 suppliers
$10.00/1g

366452-96-2
34 suppliers
$45.00/10mg
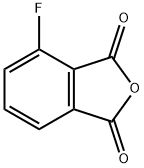
652-39-1
256 suppliers
$9.00/1g

366452-96-2
34 suppliers
$45.00/10mg
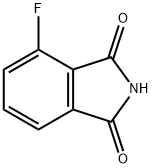
51108-29-3
45 suppliers
$31.00/100mg

366452-96-2
34 suppliers
$45.00/10mg